Timing of anti-PD-L1 antibody initiation affects efficacy/toxicity of CD19 CAR T-cell therapy for large B-cell lymphoma
- PMID: 37903325
- PMCID: PMC10837185
- DOI: 10.1182/bloodadvances.2023011287
Timing of anti-PD-L1 antibody initiation affects efficacy/toxicity of CD19 CAR T-cell therapy for large B-cell lymphoma
Abstract
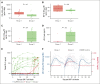
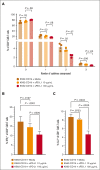
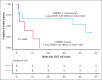
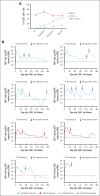
More than half of the patients treated with CD19-targeted chimeric antigen receptor (CAR) T-cell immunotherapy for large B-cell lymphoma (LBCL) do not achieve durable remission, which may be partly due to PD-1/PD-L1-associated CAR T-cell dysfunction. We report data from a phase 1 clinical trial (NCT02706405), in which adults with LBCL were treated with autologous CD19 CAR T cells (JCAR014) combined with escalating doses of the anti-PD-L1 monoclonal antibody, durvalumab, starting either before or after CAR T-cell infusion. The addition of durvalumab to JCAR014 was safe and not associated with increased autoimmune or immune effector cell-associated toxicities. Patients who started durvalumab before JCAR014 infusion had later onset and shorter duration of cytokine release syndrome and inferior efficacy, which was associated with slower accumulation of CAR T cells and lower concentrations of inflammatory cytokines in the blood. Initiation of durvalumab before JCAR014 infusion resulted in an early increase in soluble PD-L1 (sPD-L1) levels that coincided with the timing of maximal CAR T-cell accumulation in the blood. In vitro, sPD-L1 induced dose-dependent suppression of CAR T-cell effector function, which could contribute to inferior efficacy observed in patients who received durvalumab before JCAR014. Despite the lack of efficacy improvement and similar CAR T-cell kinetics early after infusion, ongoing durvalumab therapy after JCAR014 was associated with re-expansion of CAR T cells in the blood, late regression of CD19+ and CD19- tumors, and enhanced duration of response. Our results indicate that the timing of initiation of PD-L1 blockade is a key variable that affects outcomes after CD19 CAR T-cell immunotherapy for adults with LBCL.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.V.H. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company, and Nektar Therapeutics, and honoraria from Bristol Myers Squibb and Novartis. E.L.K. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company. S.F. reports grants from Bristol Myers Squibb and other support in the form of pending equity from Link Immunotherapeutics outside of the submitted work; has issued patents for PCT/US2021/025255 and PCT/US2021/025248d; and a patent for PCT/US2021/025260 issued, licensed, and with royalties paid from Bristol Myers Squibb. J.G. has received research funding from Sobi, Juno Therapeutics, a Bristol Myers Squibb company, Celgene, and Angiocrine Bioscience, and has received honoraria/consulting fees from Sobi, Legend Biotech, Janssen, Kite Pharma, a Gilead company, and MorphoSys. C.C.S.Y. has received research funding from OBI Pharma, Lonza, Sensei, Signal One, and Pfizer, and serves on scientific advisory boards for Twinstrand Biosciences, AbbVie, Eli Lilly, and Loxo. R.D.C. has received research funding from Amgen, Incyte, Kite Pharma, a Gilead company, Pfizer, Servier, and Vanda Pharmaceuticals; has received honoraria/consulting fees from Amgen, Jazz, Kite/Gilead, and Pfizer; serves on a data and safety monitoring board for Pepromene Bio and on an independent response review committee for Autolus; and his spouse has been employed by and owned stock in Seagen. A.G.C. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company. D.J.G. has received research funding from, has served as an adviser for, and has received royalties from Juno Therapeutics, a Bristol Myers Squibb company; has served as an adviser and received research funding from Seattle Genetics; has served as an adviser to GlaxoSmithKline, Celgene, Janssen Biotech, and Legend Biotech; and has received research funding from SpringWorks Therapeutics, Sanofi, and Cellectar Biosciences. M.S. has received research funding from Mustang Bio, Bristol Myers Squibb, Pharmacyclics, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; has served as a consultant for AbbVie, Genentech, AstraZeneca, Pharmacyclics, Beigene, Bristol Myers Squibb, MorphoSys/Incyte, Kite, Eli Lilly, Genmab, Mustang Bio, Regeneron, ADC Therapeutics, Janssen, Fate Therapeutics, and MEI Pharma; and his spouse is an employee of Bristol Myers Squibb. B.G.T. has received research funding from Mustang Bio and Juno Therapeutics, a Bristol Myers Squibb company; serves on scientific advisory boards for Mustang Bio and Proteios Technology; and has the right to receive royalties from Fred Hutch as an inventor on licensed patents. S.R.R. is a cofounder and adviser to Lyell Immunopharma and has received research funding from and intellectual property licensed to Lyell Immunopharma; was a cofounder of Juno Therapeutics, a Bristol Myers Squibb company; is an inventor of patents licensed to Juno Therapeutics; and served as an adviser to Juno Therapeutics and Adaptive Biotechnologies. D.G.M. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company, Celgene, and Kite Pharma, a Gilead company; has served on ad hoc advisory board meetings for Amgen, Bristol Myers Squibb, Genentech, Gilead, Incyte, Janssen, Legend Biotech, Mustang Bio, MorphoSys, Novartis, Pharmacyclics, and Umoja; has rights to receive royalties from Fred Hutch for patents licensed to Juno Therapeutics; and serves on scientific advisory board with stock options and compensations for A2 Biotherapeutics and Navan Technologies. C.J.T. has received research funding from Juno Therapeutics, a Bristol Myers Squibb company, NanoString Technologies, and Nektar Therapeutics; serves on scientific advisory boards for Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, and Cargo Therapeutics; serves on a data and safety monitoring board for Kyverna; has served on ad hoc advisory board meetings (last 12 months) for Legend Biotech, Nektar Therapeutics, and Syncopation Life Sciences; performs consulting for Century Therapeutics, Orna Therapetuics, and IGM Biosciences; has stock options in Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, Cargo Therapeutics, and ArsenalBio; and has the right to receive payments from Fred Hutch as an inventor on licensed patents. The remaining authors have declared no competing financial interests.
Figures




Comment in
-
Checkpoints: roadblocks or repairs for the CAR-T journey?Blood Adv. 2024 Jan 23;8(2):468-469. doi: 10.1182/bloodadvances.2023012078. Blood Adv. 2024. PMID: 38261329 Free PMC article. No abstract available.
References
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

