Ring Opening of Aziridines by Pendant Sulfamates Allows for Regioselective and Stereospecific Preparation of Vicinal Diamines
- PMID: 37903411
- PMCID: PMC10799289
- DOI: 10.1021/acs.joc.3c01731
Ring Opening of Aziridines by Pendant Sulfamates Allows for Regioselective and Stereospecific Preparation of Vicinal Diamines
Abstract
The ring opening of aziridines by pendant sulfamates is a viable strategy for the rapid preparation of vicinal diamines. Our reaction is compatible with both disubstituted cis- and trans-aziridines; unsubstituted, N-alkyl, and N-aryl sulfamates engage effectively. In all cases examined, the cyclization reaction is perfectly regioselective and stereospecific. Once activated, the product oxathiazinane heterocycles can be ring opened with a diverse range of nucleophiles.
Figures
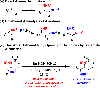


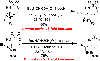

Similar articles
-
Ring-opening and -expansion of 2,2'-biaziridine: access to diverse enantiopure linear and bicyclic vicinal diamines.Org Lett. 2014 Aug 15;16(16):4344-7. doi: 10.1021/ol502164b. Epub 2014 Aug 7. Org Lett. 2014. PMID: 25101915
-
Palladium-Catalyzed Regioselective and Stereospecific Ring-Opening Cross-Coupling of Aziridines: Experimental and Computational Studies.Acc Chem Res. 2020 Aug 18;53(8):1686-1702. doi: 10.1021/acs.accounts.0c00395. Epub 2020 Aug 6. Acc Chem Res. 2020. PMID: 32786337
-
Ring Opening of Aziridines by Pendant Silanols Allows for Stereospecific Preparations of 1'-Amino-tetrahydrofurans.J Org Chem. 2023 Jul 7;88(13):9136-9156. doi: 10.1021/acs.joc.3c00763. Epub 2023 May 30. J Org Chem. 2023. PMID: 37253098 Free PMC article.
-
B2(OH)4-Mediated Reductive Ring-Opening of N-Tosyl Aziridines by Nitroarenes: A Green and Regioselective Access to Vicinal Diamines.J Org Chem. 2024 Jun 21;89(12):8656-8667. doi: 10.1021/acs.joc.4c00591. Epub 2024 Jun 3. J Org Chem. 2024. PMID: 38831644
-
Ring Opening of Aziridines by Pendant Silanols Allows for Preparations of (±)-Clavaminol H, (±)-Des-Acetyl-Clavaminol H, (±)-Dihydrosphingosine, and (±)-N-Hexanoyldihydrosphingosine.Org Lett. 2022 Aug 26;24(33):6202-6207. doi: 10.1021/acs.orglett.2c02496. Epub 2022 Aug 11. Org Lett. 2022. PMID: 35951966 Free PMC article.
Cited by
-
Ammonium Zincates as Catalysts for the Microwave-Enhanced Synthesis of Symmetric Piperazines by Regioselective Opening of Aziridines.Chem Asian J. 2024 Nov 18;19(22):e202400688. doi: 10.1002/asia.202400688. Epub 2024 Oct 18. Chem Asian J. 2024. PMID: 39136397 Free PMC article.
-
A Catalytic, Enantioselective Sulfamate Tethered Aza-Michael Cyclization.Org Lett. 2024 Dec 20;26(50):10708-10713. doi: 10.1021/acs.orglett.4c03558. Epub 2024 Dec 11. Org Lett. 2024. PMID: 39660506 Free PMC article.
-
Aziridination via Nitrogen-Atom Transfer to Olefins from Photoexcited Azoxy-Triazenes.J Am Chem Soc. 2024 Apr 10;146(14):9499-9505. doi: 10.1021/jacs.3c14713. Epub 2024 Mar 24. J Am Chem Soc. 2024. PMID: 38522088 Free PMC article.
-
Exploration of One-Pot, Tandem Sulfamoylation and aza-Michael Cyclization Reactions for the Syntheses of Oxathiazinane Dioxide Heterocycles.J Org Chem. 2024 Nov 15;89(22):16774-16778. doi: 10.1021/acs.joc.4c02086. Epub 2024 Nov 4. J Org Chem. 2024. PMID: 39492674 Free PMC article.
-
Fun With Unusual Functional Groups: Sulfamates, Phosphoramidates, and Di-tert-butyl Silanols.European J Org Chem. 2024 Mar 1;27(9):e202301283. doi: 10.1002/ejoc.202301283. Epub 2024 Jan 15. European J Org Chem. 2024. PMID: 39309710 Free PMC article.
References
-
- Bennani YL; Hanessian S, trans-1,2-Diaminocyclohexane Derivatives as Chiral Reagents, Scaffolds, and Ligands for Catalysis: Applications in Asymmetric Synthesis and Molecular Recognition. Chem. Rev. 1997, 97, 3161–3196. - PubMed
-
- Lucet D; Le Gall T; Mioskowski C, The Chemistry of Vicinal Diamines. Angew. Chem. Int. Ed. 1998, 37, 2580–2627. - PubMed
-
- Viso A; Fernández de la Pradilla R; García A; Flores A, α,β-Diamino Acids: Biological Significance and Synthetic Approaches. Chem. Rev. 2005, 105, 3167–3196. - PubMed
-
- Kizirian J-C, Chiral Tertiary Diamines in Asymmetric Synthesis. Chem. Rev. 2008, 108, 140–205. - PubMed
-
- Saibabu Kotti SRS; Timmons C; Li G, Vicinal Diamino Functionalities as Privileged Structural Elements in Biologically Active Compounds and Exploitation of their Synthetic Chemistry. Chem. Biol. Drug Des. 2006, 67, 101–114. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

