Patient Brain Organoids Identify a Link between the 16p11.2 Copy Number Variant and the RBFOX1 Gene
- PMID: 37903506
- PMCID: PMC10655044
- DOI: 10.1021/acschemneuro.3c00442
Patient Brain Organoids Identify a Link between the 16p11.2 Copy Number Variant and the RBFOX1 Gene
Abstract
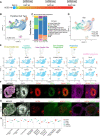
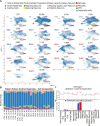
Copy number variants (CNVs) that delete or duplicate 30 genes within the 16p11.2 genomic region give rise to a range of neurodevelopmental phenotypes with high penetrance in humans. Despite the identification of this small region, the mechanisms by which 16p11.2 CNVs lead to disease are unclear. Relevant models, such as human cortical organoids (hCOs), are needed to understand the human-specific mechanisms of neurodevelopmental disease. We generated hCOs from 17 patients and controls, profiling 167,958 cells with single-cell RNA-sequencing analysis, which revealed neuronal-specific differential expression of genes outside the 16p11.2 region that are related to cell-cell adhesion, neuronal projection growth, and neurodevelopmental disorders. Furthermore, 16p11.2 deletion syndrome organoids exhibited reduced mRNA and protein levels of RBFOX1, a gene that can also harbor CNVs linked to neurodevelopmental phenotypes. We found that the genes previously shown to be regulated by RBFOX1 are also perturbed in organoids from patients with the 16p11.2 deletion syndrome and thus identified a novel link between independent CNVs associated with neuronal development and autism. Overall, this work suggests convergent signaling, which indicates the possibility of a common therapeutic mechanism across multiple rare neuronal diseases.
Keywords: 16p11.2; CNV; RBFOX1; autism; brain organoids; scRNA-seq.
Conflict of interest statement
The authors declare the following competing financial interest(s): During the time of this research, all scientists were employed by the Novartis Institutes for BioMedical Research.
Figures





References
-
- Noor A.; Lionel A. C.; Cohen-Woods S.; Moghimi N.; Rucker J.; Fennell A.; Thiruvahindrapuram B.; Kaufman L.; Degagne B.; Wei J.; Parikh S. V.; Muglia P.; Forte J.; Scherer S. W.; Kennedy J. L.; Xu W.; McGuffin P.; Farmer A.; Strauss J.; Vincent J. B. Copy Number Variant Study of Bipolar Disorder in Canadian and UK Populations Implicates Synaptic Genes. Am. J. Med. Genet. 2014, 165 (4), 303–313. 10.1002/ajmg.b.32232. - DOI - PubMed
-
- Zarrei M.; Burton C. L.; Engchuan W.; Young E. J.; Higginbotham E. J.; MacDonald J. R.; Trost B.; Chan A. J. S.; Walker S.; Lamoureux S.; Heung T.; Mojarad B. A.; Kellam B.; Paton T.; Faheem M.; Miron K.; Lu C.; Wang T.; Samler K.; Wang X.; Costain G.; Hoang N.; Pellecchia G.; Wei J.; Patel R. V.; Thiruvahindrapuram B.; Roifman M.; Merico D.; Goodale T.; Drmic I.; Speevak M.; Howe J. L.; Yuen R. K. C.; Buchanan J. A.; Vorstman J. A. S.; Marshall C. R.; Wintle R. F.; Rosenberg D. R.; Hanna G. L.; Woodbury-Smith M.; Cytrynbaum C.; Zwaigenbaum L.; Elsabbagh M.; Flanagan J.; Fernandez B. A.; Carter M. T.; Szatmari P.; Roberts W.; Lerch J.; Liu X.; Nicolson R.; Georgiades S.; Weksberg R.; Arnold P. D.; Bassett A. S.; Crosbie J.; Schachar R.; Stavropoulos D. J.; Anagnostou E.; Scherer S. W. A Large Data Resource of Genomic Copy Number Variation across Neurodevelopmental Disorders. npj Genom. Med. 2019, 4 (1), 26.10.1038/s41525-019-0098-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

