DIAPH1-MFN2 interaction regulates mitochondria-SR/ER contact and modulates ischemic/hypoxic stress
- PMID: 37903764
- PMCID: PMC10616211
- DOI: 10.1038/s41467-023-42521-x
DIAPH1-MFN2 interaction regulates mitochondria-SR/ER contact and modulates ischemic/hypoxic stress
Abstract
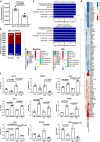
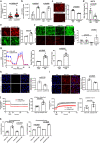
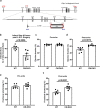
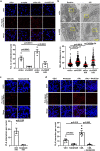
Inter-organelle contact and communication between mitochondria and sarco/endoplasmic reticulum (SR/ER) maintain cellular homeostasis and are profoundly disturbed during tissue ischemia. We tested the hypothesis that the formin Diaphanous-1 (DIAPH1), which regulates actin dynamics, signal transduction and metabolic functions, contributes to these processes. We demonstrate that DIAPH1 interacts directly with Mitofusin-2 (MFN2) to shorten mitochondria-SR/ER distance, thereby enhancing mitochondria-ER contact in cells including cardiomyocytes, endothelial cells and macrophages. Solution structure studies affirm the interaction between the Diaphanous Inhibitory Domain and the cytosolic GTPase domain of MFN2. In male rodent and human cardiomyocytes, DIAPH1-MFN2 interaction regulates mitochondrial turnover, mitophagy, and oxidative stress. Introduction of synthetic linker construct, which shorten the mitochondria-SR/ER distance, mitigated the molecular and functional benefits of DIAPH1 silencing in ischemia. This work establishes fundamental roles for DIAPH1-MFN2 interaction in the regulation of mitochondria-SR/ER contact networks. We propose that targeting pathways that regulate DIAPH1-MFN2 interactions may facilitate recovery from tissue ischemia.
© 2023. The Author(s).
Conflict of interest statement
A.M.S., A.S., and R.R. have patents and patent applications through NYU Grossman School of Medicine that have been submitted/published and that are related to some of the work detailed in this manuscript. These include the following: (i) Patent Number 10,729,695 (United States), (ii) patent number 10,265,320 (United States), (iii) application number 14851038.1 (pending, Europe), (iv) application number 16/094,720 (pending, United States), (v) application number 17786436.0 (pending, Europe), (vi) application number PCT/US2020/044926 (pending, United States). R.R. is a consultant for Applied Therapeutics. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

