Exploring intrinsic variability between cultured nasal and bronchial epithelia in cystic fibrosis
- PMID: 37903789
- PMCID: PMC10616285
- DOI: 10.1038/s41598-023-45201-4
Exploring intrinsic variability between cultured nasal and bronchial epithelia in cystic fibrosis
Abstract
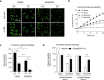
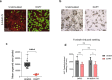
The nasal and bronchial epithelium are unified parts of the respiratory tract that are affected in the monogenic disorder cystic fibrosis (CF). Recent studies have uncovered that nasal and bronchial tissues exhibit intrinsic variability, including differences in mucociliary cell composition and expression of unique transcriptional regulatory proteins which relate to germ layer origin. In the present study, we explored whether intrinsic differences between nasal and bronchial epithelial cells persist in cell cultures and affect epithelial cell functioning in CF. Comparison of air-liquid interface (ALI) differentiated epithelial cells from subjects with CF revealed distinct mucociliary differentiation states of nasal and bronchial cultures. Moreover, using RNA sequencing we identified cell type-specific signature transcription factors in differentiated nasal and bronchial epithelial cells, some of which were already poised for expression in basal progenitor cells as evidenced by ATAC sequencing. Analysis of differentiated nasal and bronchial epithelial 3D organoids revealed distinct capacities for fluid secretion, which was linked to differences in ciliated cell differentiation. In conclusion, we show that unique phenotypical and functional features of nasal and bronchial epithelial cells persist in cell culture models, which can be further used to investigate the effects of tissue-specific features on upper and lower respiratory disease development in CF.
© 2023. The Author(s).
Conflict of interest statement
J.M.B. has a patent granted (10006904) related to CFTR function measurements in organoids and received personal fees from HUB/Royal Dutch academy of sciences, during the conduct of the study; nonfinancial support from Vertex Pharmaceuticals and personal fees and nonfinancial support from Proteostasis Therapeutics, outside the submitted work. C.K.v.d.E. reports grants from GSK, Nutricia, TEVA, Gilead, Vertex, ProQR, Proteostasis, Galapagos NV, Eloxx pharmaceuticals, outside the submitted work; In addition, C.K.v.d.E. has a patent related to CFTR function measurements in organoids (10006904) with royalties paid. The other authors declare no potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

