A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering
- PMID: 37903891
- PMCID: PMC10954421
- DOI: 10.1038/s41565-023-01533-w
A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering
Abstract
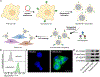
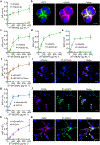
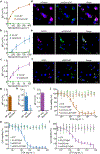
Since their initial development, cell membrane-coated nanoparticles (CNPs) have become increasingly popular in the biomedical field. Despite their inherent versatility and ability to enable complex biological applications, there is considerable interest in augmenting the performance of CNPs through the introduction of additional functionalities. Here we demonstrate a genetic-engineering-based modular approach to CNP functionalization that can encompass a wide range of ligands onto the nanoparticle surface. The cell membrane coating is engineered to express a SpyCatcher membrane anchor that can readily form a covalent bond with any moiety modified with SpyTag. To demonstrate the broad utility of this technique, three unique targeted CNP formulations are generated using different classes of targeting ligands, including a designed ankyrin repeat protein, an affibody and a single-chain variable fragment. In vitro, the modified nanoparticles exhibit enhanced affinity towards cell lines overexpressing the cognate receptors for each ligand. When formulated with a chemotherapeutic payload, the modularly functionalized nanoparticles display strong targeting ability and growth suppression in a murine tumour xenograft model of ovarian cancer. Our data suggest genetic engineering offers a feasible approach for accelerating the development of multifunctional CNPs for a broad range of biomedical applications.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
References (Methods only)
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

