Structural surfaceomics reveals an AML-specific conformation of integrin β2 as a CAR T cellular therapy target
- PMID: 37904046
- PMCID: PMC10663162
- DOI: 10.1038/s43018-023-00652-6
Structural surfaceomics reveals an AML-specific conformation of integrin β2 as a CAR T cellular therapy target
Abstract
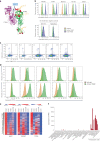
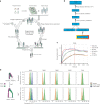
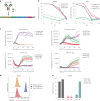
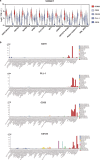
Safely expanding indications for cellular therapies has been challenging given a lack of highly cancer-specific surface markers. Here we explore the hypothesis that tumor cells express cancer-specific surface protein conformations that are invisible to standard target discovery pipelines evaluating gene or protein expression, and these conformations can be identified and immunotherapeutically targeted. We term this strategy integrating cross-linking mass spectrometry with glycoprotein surface capture 'structural surfaceomics'. As a proof of principle, we apply this technology to acute myeloid leukemia (AML), a hematologic malignancy with dismal outcomes and no known optimal immunotherapy target. We identify the activated conformation of integrin β2 as a structurally defined, widely expressed AML-specific target. We develop and characterize recombinant antibodies to this protein conformation and show that chimeric antigen receptor T cells eliminate AML cells and patient-derived xenografts without notable toxicity toward normal hematopoietic cells. Our findings validate an AML conformation-specific target antigen and demonstrate a tool kit for applying these strategies more broadly.
© 2023. The Author(s).
Conflict of interest statement
K.M., J.J.A., S.S.S. and A.P.W. have filed a patent related to the antibody sequences described herein. A.P.W. has received research funding from Genentech. C.S. has received research funding from Revolution Medicines, Abbvie and Erasca and has served on advisory boards for Genentech, Abbvie and Astellas. C.M.R. has equity in Allovir and Marker Therapeutics, has received research support from Tessa Therapeutics and is on the Scientific Advisory Board of Tessa Therapeutics and Marker. The spouse of C.M.R. has served on advisory boards for Walking Fish Therapeutics, CellGenix, Marker Therapeutics, Tessa Therapeutics, Abintus, Allogene, Bellicum Pharmaceuticals, Bluebird Bio, Athenex, Memgen, Turnstone Biologics, Coya Therapeutics, TScan Therapeutics, Onkimmune, Poseida Therapeutics and Allovir. The other authors declare no competing interests.
Figures






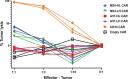
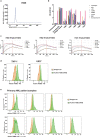
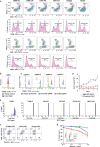
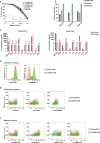

References
-
- Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017;16:315–337. - PubMed
-
- Hosen N, et al. The activated conformation of integrin β7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat. Med. 2017;23:1436–1443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

