Hypoxia enhances anti-fibrotic properties of extracellular vesicles derived from hiPSCs via the miR302b-3p/TGFβ/SMAD2 axis
- PMID: 37904135
- PMCID: PMC10617123
- DOI: 10.1186/s12916-023-03117-w
Hypoxia enhances anti-fibrotic properties of extracellular vesicles derived from hiPSCs via the miR302b-3p/TGFβ/SMAD2 axis
Abstract
Background: Cardiac fibrosis is one of the top killers among fibrotic diseases and continues to be a global unaddressed health problem. The lack of effective treatment combined with the considerable socioeconomic burden highlights the urgent need for innovative therapeutic options. Here, we evaluated the anti-fibrotic properties of extracellular vesicles (EVs) derived from human induced pluripotent stem cells (hiPSCs) that were cultured under various oxygen concentrations.
Methods: EVs were isolated from three hiPSC lines cultured under normoxia (21% O2; EV-N) or reduced oxygen concentration (hypoxia): 3% O2 (EV-H3) or 5% O2 (EV-H5). The anti-fibrotic activity of EVs was tested in an in vitro model of cardiac fibrosis, followed by a detailed investigation of the underlying molecular mechanisms. Sequencing of EV miRNAs combined with bioinformatics analysis was conducted and a selected miRNA was validated using a miRNA mimic and inhibitor. Finally, EVs were tested in a mouse model of angiotensin II-induced cardiac fibrosis.
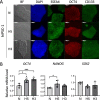
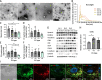
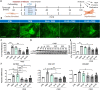
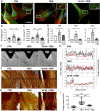
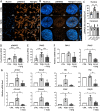
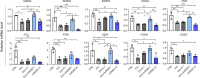
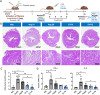
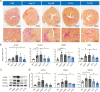
Results: We provide evidence that an oxygen concentration of 5% enhances the anti-fibrotic effects of hiPS-EVs. These EVs were more effective in reducing pro-fibrotic markers in activated human cardiac fibroblasts, when compared to EV-N or EV-H3. We show that EV-H5 act through the canonical TGFβ/SMAD pathway, primarily via miR-302b-3p, which is the most abundant miRNA in EV-H5. Our results show that EV-H5 not only target transcripts of several profibrotic genes, including SMAD2 and TGFBR2, but also reduce the stiffness of activated fibroblasts. In a mouse model of heart fibrosis, EV-H5 outperformed EV-N in suppressing the inflammatory response in the host and by attenuating collagen deposition and reducing pro-fibrotic markers in cardiac tissue.
Conclusions: In this work, we provide evidence of superior anti-fibrotic properties of EV-H5 over EV-N or EV-H3. Our study uncovers that fine regulation of oxygen concentration in the cellular environment may enhance the anti-fibrotic effects of hiPS-EVs, which has great potential to be applied for heart regeneration.
Keywords: Extracellular vesicles; Heart fibrosis; Hypoxia; Induced pluripotent stem cells; Low oxygen; Therapy.
© 2023. The Author(s).
Conflict of interest statement
Jagiellonian University has filed a patent application for the use of hiPS-EVs derived from oxygen conditions of 5% on behalf of the inventors: S.B.-W., M.Paw (M.P.1), A.A.K., J.J.L., M.Piejko (M.P.2), D.W., K.B.S., and P.E.F. The remaining authors declare no competing interests in relation to this work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

