This is a preprint.
Dynamics of SARS-CoV-2 Seroprevalence in a Large US population Over a Period of 12 Months
- PMID: 37904956
- PMCID: PMC10614993
- DOI: 10.1101/2023.10.20.23297329
Dynamics of SARS-CoV-2 Seroprevalence in a Large US population Over a Period of 12 Months
Abstract
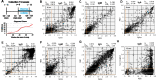
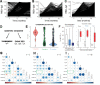
Due to a combination of asymptomatic or undiagnosed infections, the proportion of the United States population infected with SARS-CoV-2 was unclear from the beginning of the pandemic. We previously established a platform to screen for SARS-CoV-2 positivity across a representative proportion of the US population, from which we reported that almost 17 million Americans were estimated to have had undocumented infections in the Spring of 2020. Since then, vaccine rollout and prevalence of different SARS-CoV-2 variants have further altered seropositivity trends within the United States population. To explore the longitudinal impacts of the pandemic and vaccine responses on seropositivity, we re-enrolled participants from our baseline study in a 6- and 12- month follow-up study to develop a longitudinal antibody profile capable of representing seropositivity within the United States during a critical period just prior to and during the initiation of vaccine rollout. Initial measurements showed that, since July 2020, seropositivity elevated within this population from 4.8% at baseline to 36.2% and 89.3% at 6 and 12 months, respectively. We also evaluated nucleocapsid seropositivity and compared to spike seropositivity to identify trends in infection versus vaccination relative to baseline. These data serve as a window into a critical timeframe within the COVID-19 pandemic response and serve as a resource that could be used in subsequent respiratory illness outbreaks.
Figures





References
-
- W. H. Organization. (WHO, 2023).
-
- Bobrovitz N. et al. , Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. MedRxiv, 2020.2005.2010.20097451 (2020).
-
- Duarte N. et al. , in Open forum infectious diseases. (Oxford University Press US, 2022), vol. 9, pp. ofab632.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
