This is a preprint.
Exercise Training and Cold Exposure Trigger Distinct Molecular Adaptations to Inguinal White Adipose Tissue
- PMID: 37905018
- PMCID: PMC10614850
- DOI: 10.1101/2023.10.16.562635
Exercise Training and Cold Exposure Trigger Distinct Molecular Adaptations to Inguinal White Adipose Tissue
Update in
-
Exercise training and cold exposure trigger distinct molecular adaptations to inguinal white adipose tissue.Cell Rep. 2024 Jul 23;43(7):114481. doi: 10.1016/j.celrep.2024.114481. Epub 2024 Jul 13. Cell Rep. 2024. PMID: 39003734 Free PMC article.
Abstract
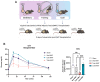
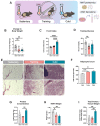
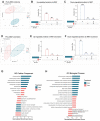
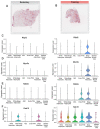
Exercise training and cold exposure both improve systemic metabolism, but the mechanisms are not well-established. We tested the hypothesis that adaptations to inguinal white adipose tissue (iWAT) are critical for these beneficial effects by determining the impact of exercise-trained and cold-exposed iWAT on systemic glucose metabolism and the iWAT proteome and secretome. Transplanting trained iWAT into sedentary mice improved glucose tolerance, while cold-exposed iWAT transplantation showed no such benefit. Compared to training, cold led to more pronounced alterations in the iWAT proteome and secretome, downregulating >2,000 proteins but also boosting iWAT's thermogenic capacity. In contrast, only training increased extracellular space and vesicle transport proteins, and only training upregulated proteins that correlate with favorable fasting glucose, suggesting fundamental changes in trained iWAT that mediate tissue-to-tissue communication. This study defines the unique exercise training- and cold exposure-induced iWAT proteomes, revealing distinct mechanisms for the beneficial effects of these interventions on metabolic health.
Keywords: adipose tissue; cold; exercise; proteomics; secretome; transplantation.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests. R.J.W.M. and L.J.G. have received research support from Novo Nordisk, which is unrelated to this work.
Figures

References
-
- Nigro P., Vamvini M., Yang J., Caputo T., Ho L.-L., Carbone N.P., Papadopoulos D., Conlin R., He J., Hirshman M.F., et al. (2023). Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix. Cell Rep. 42, 112392. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
