This is a preprint.
Multi-pass, single-molecule nanopore reading of long protein strands with single-amino acid sensitivity
- PMID: 37905023
- PMCID: PMC10614977
- DOI: 10.1101/2023.10.19.563182
Multi-pass, single-molecule nanopore reading of long protein strands with single-amino acid sensitivity
Update in
-
Multi-pass, single-molecule nanopore reading of long protein strands.Nature. 2024 Sep;633(8030):662-669. doi: 10.1038/s41586-024-07935-7. Epub 2024 Sep 11. Nature. 2024. PMID: 39261738 Free PMC article.
Abstract
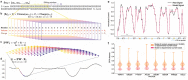
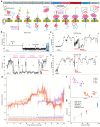
The ability to sequence single protein molecules in their native, full-length form would enable a more comprehensive understanding of proteomic diversity. Current technologies, however, are limited in achieving this goal. Here, we establish a method for long-range, single-molecule reading of intact protein strands on a commercial nanopore sensor array. By using the ClpX unfoldase to ratchet proteins through a CsgG nanopore, we achieve single-amino acid level sensitivity, enabling sequencing of combinations of amino acid substitutions across long protein strands. For greater sequencing accuracy, we demonstrate the ability to reread individual protein molecules, spanning hundreds of amino acids in length, multiple times, and explore the potential for high accuracy protein barcode sequencing. Further, we develop a biophysical model that can simulate raw nanopore signals a priori, based on amino acid volume and charge, enhancing the interpretation of raw signal data. Finally, we apply these methods to examine intact, folded protein domains for complete end-to-end analysis. These results provide proof-of-concept for a platform that has the potential to identify and characterize full-length proteoforms at single-molecule resolution.
Conflict of interest statement
Competing interests: Provisional patents covering aspects of this work have been filed by the University of Washington. JN is a consultant to Oxford Nanopore Technologies. The remaining authors declare no competing interests.
Figures




References
-
- Strahl B. D. & Allis C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
