This is a preprint.
Differential effects of follicle-stimulating hormone glycoforms on the transcriptome profile of cultured rat granulosa cells as disclosed by RNA-seq
- PMID: 37905087
- PMCID: PMC10614937
- DOI: 10.1101/2023.10.18.562995
Differential effects of follicle-stimulating hormone glycoforms on the transcriptome profile of cultured rat granulosa cells as disclosed by RNA-seq
Update in
-
Differential effects of follicle-stimulating hormone glycoforms on the transcriptome profile of cultured rat granulosa cells as disclosed by RNA-seq.PLoS One. 2024 Jun 6;19(6):e0293688. doi: 10.1371/journal.pone.0293688. eCollection 2024. PLoS One. 2024. PMID: 38843139 Free PMC article.
Abstract
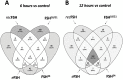
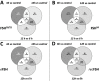
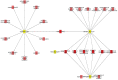
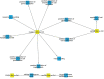
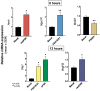
It has been documented that variations in glycosylation on glycoprotein hormones, confer distinctly different biological features to the corresponding glycoforms when multiple in vitro biochemical readings are analyzed. We here applied next generation RNA sequencing to explore changes in the transcriptome of rat granulosa cells exposed for 0, 6, and 12 h to 100 ng/ml of four highly purified follicle-stimulating hormone (FSH) glycoforms, each exhibiting different glycosylation patterns: human pituitary FSH18/21 and equine FSH (eqFSH) (hypo-glycosylated), and human FSH24 and chinese-hamster ovary cell-derived human recombinant FSH (recFSH) (fully-glycosylated). Total RNA from triplicate incubations was prepared from FSH glycoform-exposed cultured granulosa cells obtained from DES-pretreated immature female rats, and RNA libraries were sequenced in a HighSeq 2500 sequencer (2 × 125 bp paired-end format, 10-15 × 106 reads/sample). The computational workflow focused on investigating differences among the four FSH glycoforms at three levels: gene expression, enriched biological processes, and perturbed pathways. Among the top 200 differentially expressed genes, only 4 (0.6%) were shared by all 4 glycoforms at 6 h, whereas 118 genes (40%) were shared at 12 h. Follicle-stimulating hormone glycocoforms stimulated different patterns of exclusive and associated up regulated biological processes in a glycoform and time-dependent fashion with more shared biological processes after 12 h of exposure and fewer treatment-specific ones, except for recFSH, which exhibited stronger responses with more specifically associated processes at this time. Similar results were found for down-regulated processes, with a greater number of processes at 6 h or 12 h, depending on the particular glycoform. In general, there were fewer downregulated than upregulated processes at both 6 h and 12 h, with FSH18/21 exhibiting the largest number of down-regulated associated processes at 6 h while eqFSH exhibited the greatest number at 12 h. Signaling cascades, largely linked to cAMP-PKA, MAPK, and PI3/AKT pathways were detected as differentially activated by the glycoforms, with each glycoform exhibiting its own molecular signature. These data extend previous observations demonstrating glycosylation-dependent differential regulation of gene expression and intracellular signaling pathways triggered by FSH in granulosa cells. The results also suggest the importance of individual FSH glycoform glycosylation for the conformation of the ligand-receptor complex and induced signalling pathways.
Keywords: Differential gene expression; Follicle-stimulating hormone glycoforms; Next Generation sequencing; RNA-seq; Transcriptome.
Figures


Similar articles
-
Differential effects of follicle-stimulating hormone glycoforms on the transcriptome profile of cultured rat granulosa cells as disclosed by RNA-seq.PLoS One. 2024 Jun 6;19(6):e0293688. doi: 10.1371/journal.pone.0293688. eCollection 2024. PLoS One. 2024. PMID: 38843139 Free PMC article.
-
Hypo-glycosylated hFSH drives ovarian follicular development more efficiently than fully-glycosylated hFSH: enhanced transcription and PI3K and MAPK signaling.Hum Reprod. 2021 Jun 18;36(7):1891-1906. doi: 10.1093/humrep/deab135. Hum Reprod. 2021. PMID: 34059912 Free PMC article.
-
In Vitro Impact of FSH Glycosylation Variants on FSH Receptor-stimulated Signal Transduction and Functional Selectivity.J Endocr Soc. 2020 Feb 18;4(5):bvaa019. doi: 10.1210/jendso/bvaa019. eCollection 2020 May 1. J Endocr Soc. 2020. PMID: 32342021 Free PMC article.
-
Biased signaling by human follicle-stimulating hormone variants.Pharmacol Ther. 2025 Apr;268:108821. doi: 10.1016/j.pharmthera.2025.108821. Epub 2025 Feb 15. Pharmacol Ther. 2025. PMID: 39961417 Review.
-
Assessment of the in vitro and in vivo biological activities of the human follicle-stimulating isohormones.Mol Cell Endocrinol. 2002 Jan 25;186(2):189-98. doi: 10.1016/s0303-7207(01)00657-8. Mol Cell Endocrinol. 2002. PMID: 11900895 Review.
References
-
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–95. - PubMed
-
- Bousfield GR, Butnev VY, Gotschall RR, Baker VL, Moore WT. Structural features of mammalian gonadotropins. Mol Cell Endocrinol. 1996;125(1–2):3–19. - PubMed
-
- Sairam MR. Role of carbohydrates in glycoprotein hormone signal transduction. Faseb J. 1989;3(8):1915–26. - PubMed
-
- Sairam MR, Bhargavi GN. A role for glycosylation of the alpha subunit in transduction of biological signal in glycoprotein hormones. Science. 1985;229(4708):65–7. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
