This is a preprint.
Nanomechanics of wild-type and mutant dimers of the tip-link protein protocadherin 15
- PMID: 37905108
- PMCID: PMC10614884
- DOI: 10.1101/2023.10.17.562769
Nanomechanics of wild-type and mutant dimers of the tip-link protein protocadherin 15
Update in
-
Nanomechanics of wild-type and mutant dimers of the inner-ear tip-link protein protocadherin 15.Proc Natl Acad Sci U S A. 2024 Oct;121(40):e2404829121. doi: 10.1073/pnas.2404829121. Epub 2024 Sep 19. Proc Natl Acad Sci U S A. 2024. PMID: 39298473 Free PMC article.
Abstract
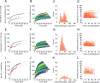
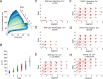
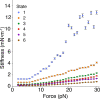
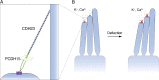
Mechanical force controls the opening and closing of mechanosensitive ion channels atop the hair bundles of the inner ear. The filamentous tip link connecting transduction channels to the tallest neighboring stereocilium modulates the force transmitted to the channels and thus changes their probability of opening. Each tip link comprises four molecules: a dimer of protocadherin 15 and a dimer of cadherin 23, all of which are stabilized by Ca2+ binding. Using a high-speed optical trap to examine dimeric PCDH15, we find that the protein's configuration is sensitive to Ca2+ and that the molecule exhibits limited unfolding at a physiological Ca2+ concentration. PCDH15 can therefore modulate its stiffness without undergoing large unfolding events in physiological Ca2+ conditions. The experimentally determined stiffness of PCDH15 accords with published values for the stiffness of the gating spring, the mechanical element that controls the opening of mechanotransduction channels. When PCDH15 has a point mutation, V507D, associated with non-syndromic hearing loss, unfolding events occur more frequently under tension and refolding events occur less often than in the wild-type protein. Our results suggest that the maintenance of appropriate tension in the gating spring is critical to the appropriate transmission of force to transduction channels, and hence to hearing.
Figures


References
-
- Kandel Eric, Koester John & Mack S. Principles of Neural Science. (McGraw Hill/Medical, 2021).
-
- Kazmierczak P. et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91 (2007). - PubMed
-
- Assad J. A., Shepherd G. M. & Corey D. P. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994 (1991). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
