Preclinical efficacy of a cell division protein candidate gonococcal vaccine identified by artificial intelligence
- PMID: 37905891
- PMCID: PMC10746169
- DOI: 10.1128/mbio.02500-23
Preclinical efficacy of a cell division protein candidate gonococcal vaccine identified by artificial intelligence
Abstract
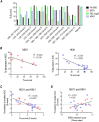
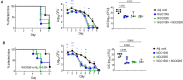
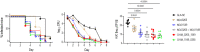
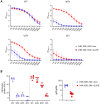
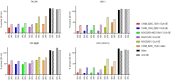
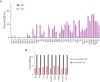
Vaccines to curb the global spread of multidrug-resistant gonorrhea are urgently needed. Here, 26 vaccine candidates identified by an artificial intelligence-driven platform (Efficacy Discriminative Educated Network[EDEN]) were screened for efficacy in the mouse vaginal colonization model. Complement-dependent bactericidal activity of antisera and the EDEN protective scores both correlated positively with the reduction in overall bacterial colonization burden. NGO1549 (FtsN) and NGO0265, both involved in cell division, displayed the best activity and were selected for further development. Both antigens, when fused to create a chimeric protein, elicited bactericidal antibodies against a wide array of gonococcal isolates and significantly attenuated the duration and burden of gonococcal colonization of mouse vaginas. Protection was abrogated in mice that lacked complement C9, the last step in the formation of the membrane attack complex pore, suggesting complement-dependent bactericidal activity as a mechanistic correlate of protection of the vaccine. FtsN and NGO0265 represent promising vaccine candidates against gonorrhea.
Keywords: Neisseria gonorrhoeae; artificial intelligence; complement; vaccine.
Conflict of interest statement
A.H.M., P.C., and S.S. are employees of EVAXION Biotech and have shares and/or stock options in the company. S.R. and P.A.R. are cofounders of STIRx, Inc., and have equity in the company.
Figures



References
-
- Centers for Disease Control and Prevention . 2023. Sexually tansmitted diseases surveillance, 2021. Department of Health and Human Services. Available from: https://www.cdc.gov/std/statistics/2021/default.htm
-
- Wang B, Giles L, Andraweera P, McMillan M, Almond S, Beazley R, Mitchell J, Lally N, Ahoure M, Denehy E, Koehler A, Flood L, Marshall H. 2022. Effectiveness and impact of the 4CMenB vaccine against invasive serogroup b meningococcal disease and gonorrhoea in an infant, child, and adolescent programme: an observational cohort and case-control study. Lancet Infect Dis 22:1011–1020. doi: 10.1016/S1473-3099(21)00754-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

