Establishing a Mouse Model of Chlorpromazine-Induced Corneal Trigeminal Denervation
- PMID: 37906054
- PMCID: PMC10619696
- DOI: 10.1167/tvst.12.10.21
Establishing a Mouse Model of Chlorpromazine-Induced Corneal Trigeminal Denervation
Abstract
Purpose: This study aimed to establish a mouse model of chlorpromazine-induced corneal trigeminal denervation (CCTD).
Methods: Retrobulbar chlorpromazine injections were administered to 6- to 8-week-old C57BL/6j mice to induce corneal denervation. Additionally, apoptosis was assessed in isolated primary trigeminal ganglion cells after culturing in a conditioned medium containing chlorpromazine. Finally, the success rate of model generation, mortality and complication rates, and model-preparation learning curves were compared between the CCTD model and the electrocoagulation and axotomy models.
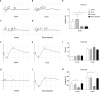
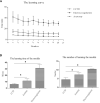
Results: Chlorpromazine retrobulbar injections resulted in trigeminal denervation, leading to a reduced blink reflex, corneal nerve density, and corneal epithelium thickness. Furthermore, 90% (9/10) of the mice developed epithelial defects, accompanied by increased apoptosis and inhibited proliferation of corneal epithelial cells. In vitro, trigeminal ganglion cell apoptosis increased after culturing in a conditioned medium containing chlorpromazine. Moreover, the CCTD model exhibited a higher success rate, longer survival rate, and lower complication rate compared to the electrocoagulation and axotomy models. Crucially, the learning curve demonstrated that the method used to generate the CCTD model was easy to learn.
Conclusions: The CCTD model is a user-friendly mouse model for studying corneal trigeminal denervation that offers a less invasive alternative to existing models.
Translational relevance: The CCTD model serves as a valuable tool for investigating the functional mechanisms of corneal trigeminal nerves and their interactions with corneal cells.
Conflict of interest statement
Disclosure:
Figures




Similar articles
-
A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain.Invest Ophthalmol Vis Sci. 2011 Apr 19;52(5):2532-9. doi: 10.1167/iovs.10-5688. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21071731 Free PMC article.
-
Corneal Denervation Causes Epithelial Apoptosis Through Inhibiting NAD+ Biosynthesis.Invest Ophthalmol Vis Sci. 2019 Aug 1;60(10):3538-3546. doi: 10.1167/iovs.19-26909. Invest Ophthalmol Vis Sci. 2019. PMID: 31415077
-
Dependence of corneal stem/progenitor cells on ocular surface innervation.Invest Ophthalmol Vis Sci. 2012 Feb 21;53(2):867-72. doi: 10.1167/iovs.11-8438. Invest Ophthalmol Vis Sci. 2012. PMID: 22232434 Free PMC article.
-
Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy.PLoS One. 2013 Aug 14;8(8):e70908. doi: 10.1371/journal.pone.0070908. eCollection 2013. PLoS One. 2013. PMID: 23967133 Free PMC article.
-
Corneal Cells: Fine-tuning Nerve Regeneration.Curr Eye Res. 2020 Mar;45(3):291-302. doi: 10.1080/02713683.2019.1675175. Epub 2019 Oct 18. Curr Eye Res. 2020. PMID: 31566418 Review.
References
-
- Lasagni Vitar RM, Rama P, Ferrari G. The two-faced effects of nerves and neuropeptides in corneal diseases. Prog Retin Eye Res. 2022; 86: 100974. - PubMed
-
- Medeiros CS, Santhiago MR.. Corneal nerves anatomy, function, injury and regeneration. Exp Eye Res. 2020; 200: 108243. - PubMed
-
- Ruiz-Lozano RE, Hernandez-Camarena JC, Loya-Garcia D, Merayo-Lloves J, Rodriguez-Garcia A. The molecular basis of neurotrophic keratopathy: diagnostic and therapeutic implications. A review. Ocul Surf. 2021; 19: 224–240. - PubMed
-
- Kowtharapu BS, Stachs O.. Corneal cells: fine-tuning nerve regeneration. Curr Eye Res. 2020; 45: 291–302. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

