First-in-human therapy with Treg produced from thymic tissue (thyTreg) in a heart transplant infant
- PMID: 37906166
- PMCID: PMC10619578
- DOI: 10.1084/jem.20231045
First-in-human therapy with Treg produced from thymic tissue (thyTreg) in a heart transplant infant
Abstract
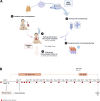
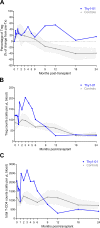
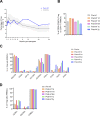
Due to their suppressive capacity, regulatory T cells (Tregs) have attracted growing interest as an adoptive cellular therapy for the prevention of allograft rejection, but limited Treg recovery and lower quality of adult-derived Tregs could represent an obstacle to success. To address this challenge, we developed a new approach that provides large quantities of Tregs with high purity and excellent features, sourced from thymic tissue routinely removed during pediatric cardiac surgeries (thyTregs). We report on a 2-year follow-up of the first patient treated worldwide with thyTregs, included in a phase I/II clinical trial evaluating the administration of autologous thyTreg in infants undergoing heart transplantation. In addition to observing no adverse effects that could be attributed to thyTreg administration, we report that the Treg frequency in the periphery was preserved during the 2-year follow-up period. These initial results are consistent with the trial objective, which is to confirm safety of the autologous thyTreg administration and its capacity to restore the Treg pool.
© 2023 Bernaldo-de-Quirós et al.
Conflict of interest statement
Disclosures: E. Bernaldo-de-Quirós reported a patent to WO2019/166658A1 pending. M. Pion reported a patent to WO2019/166658A1 issued. R. Correa-Rocha reported personal fees from THYTECH outside the submitted work; in addition, R. Correa-Rocha had a patent to WO2019/166658A1 pending. No other disclosures were reported.
Figures




References
-
- Arroyo Hornero, R., Betts G.J., Sawitzki B., Vogt K., Harden P.N., and Wood K.J.. 2017. CD45RA distinguishes CD4+CD25+CD127-/low TSDR demethylated regulatory T cell subpopulations with differential stability and susceptibility to tacrolimus-mediated inhibition of suppression. Transplantation. 101:302–309. 10.1097/TP.0000000000001278 - DOI - PMC - PubMed
-
- Bernaldo-de-Quirós, E., Cózar B., López-Esteban R., Clemente M., Gil-Jaurena J.M., Pardo C., Pita A., Pérez-Caballero R., Camino M., Gil N., et al. 2022a. A novel GMP protocol to produce high-quality Treg cells from the pediatric thymic tissue to Be employed as cellular therapy. Front. Immunol. 13:893576. 10.3389/fimmu.2022.893576 - DOI - PMC - PubMed
-
- Bernaldo-de-Quirós, E., López-Abente J., Camino M., Gil N., Panadero E., López-Esteban R., Martínez-Bonet M., Pion M., and Correa-Rocha R.. 2021. The presence of a marked imbalance between regulatory T cells and effector T cells reveals that tolerance mechanisms could Be compromised in heart transplant children. Transpl. Direct. 7:e693. 10.1097/TXD.0000000000001152 - DOI - PMC - PubMed
-
- Brunstein, C.G., Miller J.S., McKenna D.H., Hippen K.L., DeFor T.E., Sumstad D., Curtsinger J., Verneris M.R., MacMillan M.L., Levine B.L., et al. 2016. Umbilical cord blood-derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood. 127:1044–1051. 10.1182/blood-2015-06-653667 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

