Outcomes Following Early Postoperative Adjuvant Radiosurgery for Brain Metastases
- PMID: 37906192
- PMCID: PMC10618851
- DOI: 10.1001/jamanetworkopen.2023.40654
Outcomes Following Early Postoperative Adjuvant Radiosurgery for Brain Metastases
Abstract
Importance: Adjuvant stereotactic radiosurgery (SRS) enhances the local control of resected brain metastases (BrM). However, the risks of local failure (LF) and potential for posttreatment adverse radiation effects (PTRE) after early postoperative adjuvant SRS have not yet been established.
Objective: To evaluate whether adjuvant SRS delivered within a median of 14 days after surgery is associated with improved LF without a concomitant increase in PTRE.
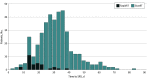
Design, setting, and participants: This prospective cohort study examines a clinical workflow (RapidRT) that was implemented from 2019 to 2022 to deliver SRS to surgical patients within a median of 14 days, ensuring all patients were treated within 30 days postoperatively. This prospective cohort was compared with a historical cohort (StanRT) of patients with BrM resected between 2013 and 2019 to assess the association of the RapidRT workflow with LF and PTRE. The 2 cohorts were combined to identify optimal SRS timing, with a median follow-up of 3.3 years for survivors.
Exposure: Timing of adjuvant SRS (14, 21, and 30 days postoperatively).
Main outcomes and measures: LF and PTRE, according to modified Response Assessment in Neuro-Oncology Brain Metastases criteria.
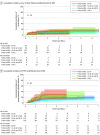
Results: There were 438 patients (265 [60.5%] female patients; 23 [5.3%] Asian, 27 [6.2%] Black, and 364 [83.1%] White patients) with a mean (SD) age of 62 (13) years; 377 were in the StanRT cohort and 61 in the RapidRT cohort. LF and PTRE rates at 1 year were not significantly different between RapidRT and StanRT cohorts. Timing of SRS was associated with radiographic PTRE. Patients receiving radiation within 14 days had the highest 1-year PTRE rate (18.08%; 95% CI, 8.31%-30.86%), and patients receiving radiation between 22 and 30 days had the lowest 1-year PTRE rate (4.10%; 95% CI, 1.52%-8.73%; P = .03). LF rates were highest for patients receiving radiation more than 30 days from surgery (10.65%; 95% CI, 6.90%-15.32%) but comparable for patients receiving radiation within 14 days, between 15 and 21 days, and between 22 and 30 days (≤14 days: 5.12%; 95% CI, 0.86%-15.60%; 15 to ≤21 days: 3.21%; 95% CI, 0.59%-9.99%; 22 to ≤30 days: 6.58%; 95% CI, 3.06%-11.94%; P = .20).
Conclusions and relevance: In this cohort study of adjuvant SRS timing following surgical resection of BrM, the optimal timing for adjuvant SRS appears to be within 22 to 30 days following surgery. The findings of this study suggest that this timing allows for a balanced approach that minimizes the risks associated with LF and PTRE.
Conflict of interest statement
Figures

References
-
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049-1060. doi: 10.1016/S1470-2045(17)30441-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

