Long-Term Safety of Guselkumab in Patients with Psoriatic Disease: An Integrated Analysis of Eleven Phase II/III Clinical Studies in Psoriasis and Psoriatic Arthritis
- PMID: 37906417
- PMCID: PMC10764399
- DOI: 10.1007/s40264-023-01361-w
Long-Term Safety of Guselkumab in Patients with Psoriatic Disease: An Integrated Analysis of Eleven Phase II/III Clinical Studies in Psoriasis and Psoriatic Arthritis
Abstract
Introduction: The benefit/risk profiles of biologics can be affected by comorbidities, certain demographic characteristics, and concomitant medications; therefore, it is important to evaluate the long-term safety profiles of biologics across broad patient populations. Guselkumab was well tolerated and efficacious across individual pivotal clinical studies in adults with moderate-to-severe psoriasis and/or active psoriatic arthritis (PsA).
Objectives: The objective of the current analysis was to evaluate guselkumab safety in a large population of patients with psoriatic disease by pooling adverse event (AE) data from 11 phase II/III studies (seven in psoriasis; four in PsA).
Methods: Guselkumab was generally administered as 100 mg subcutaneous injections at Week 0, Week 4, then every 8 weeks (Q8W) in psoriasis studies and at Week 0, Week 4, then every 4 weeks (Q4W) or Q8W in PsA studies. Safety data were summarized for the placebo-controlled period (Weeks 0-16 in psoriasis; Weeks 0-24 in PsA) and through the end of the reporting period (up to 5 years in psoriasis; up to 2 years in PsA). Using the integrated data, incidence rates of key AEs were determined post hoc, adjusted for duration of follow-up, and reported per 100 patient-years (PYs). AE rates were also determined in subgroups of patients defined by sex, age, body mass index (BMI), and prior biologic use.
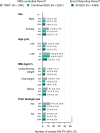
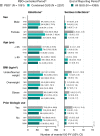
Results: During the placebo-controlled period, 1061 patients received placebo (395 PYs) and 2257 received guselkumab (856 PYs). Through the end of the reporting period, 4399 guselkumab-treated patients contributed 10,787 PYs of follow-up. During the placebo-controlled period, in the guselkumab and placebo groups, respectively, rates of AEs were 281 versus 272/100 PYs, and infections were 76.0 versus 72.2/100 PYs. Rates of serious AEs (5.6 vs. 7.8/100 PYs), AEs leading to discontinuation (4.9 vs. 6.6/100 PYs), serious infections (1.0 vs. 2.3/100 PYs), malignancy (0.59 vs. 0.25 patients/100 PYs), and major adverse cardiovascular events (MACE; 0.35 vs. 0.25/100 PYs) were low and comparable between guselkumab and placebo. Among guselkumab-treated patients, safety event rates through the end of the reporting period were numerically lower than or comparable with rates observed during the placebo-controlled period: AEs, 164/100 PYs; infections, 61.2/100 PYs; serious AEs, 5.4/100 PYs; AEs leading to discontinuation, 1.8/100 PYs; serious infections, 1.0/100 PYs; malignancy, 0.6/100 PYs; and MACE, 0.3/100 PYs. No AEs of Crohn's disease, ulcerative colitis, or active tuberculosis were reported among guselkumab-treated patients. In the psoriasis studies, no opportunistic infections were reported among guselkumab-treated patients. Three AEs of opportunistic infections were reported in guselkumab-treated patients with PsA (0.14/100 PYs; all after Week 52 in DISCOVER-2). AE rates were largely consistent across subgroups of guselkumab-treated patients defined by sex, age, BMI, and prior biologic use.
Conclusions: In this analysis of 4399 guselkumab-treated patients with psoriatic disease followed for 10,787 PYs, guselkumab had a favorable AE profile. AE rates were similar between guselkumab- and placebo-treated patients and were consistent throughout long-term guselkumab treatment and across broad subgroups of patients with psoriatic disease.
Clinical trials registrations: Clinicaltrials.gov identifiers: NCT01483599, NCT02207231, NCT02207244, NCT02203032, NCT02905331, NCT03090100, NCT02325219, NCT02319759, NCT03162796, NCT03158285, and NCT03796858.
© 2023. The Author(s).
Conflict of interest statement
Bruce Strober has served as a consultant (honoraria) and/or speaker and/or investigator for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Connect Biopharma, CorEvitas Psoriasis Registry, Dermavant, Dermira, Eli Lilly, EPI Health, Evelo Biosciences, Immunic Therapeutics, Incyte, Janssen, LEO Pharma, Maruho, Meiji Seika Pharma, Mindera Health, Novartis, Ono, Pfizer, Regeneron, Sanofi-Genzyme, Sun Pharma, UCB Pharma, Union Therapeutics, Ventyxbio, and vTv Therapeutics; and served as Co-Scientific Director (consulting fee) of CorEvitas (formerly Corrona) Psoriasis Registry and Editor-in-Chief (honorarium) of the Journal of Psoriasis and Psoriatic Arthritis. Laura C. Coates has received grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB; worked as a paid consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, Janssen, MoonLake, Novartis, Pfizer, and UCB; and has been paid as a speaker for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Medac, Novartis, Pfizer, and UCB. Dr. Coates is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and are not necessarily those of the NHS, the NIHR, or the Department of Health. Mark G. Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Inozyme, Janssen Research & Development, LLC, Ortho Dermatologics, Pfizer, Sanofi-Regeneron, and UCB, Inc., and is a consultant for Almirall, AltruBio Inc., AnaptysBio, Apogee, Arcutis, Inc., AstraZeneca, Atomwise, Avotres Therapeutics, Brickell Biotech, Boehringer Ingelheim, Bristol Myers Squibb, Castle Biosciences, Celltrion, CorEvitas, Dermavant Sciences, EPI, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Galderma, Genentech, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. Atul Deodhar has received consulting fees for participation in advisory boards from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB; research grant funding from AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB; and speaker fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and UCB. Evan Leibowitz, Katelyn Rowland, Soumya D. Chakravarty, and Daphne Chan are employees of Janssen Scientific Affairs, LLC (a subsidiary of Johnson & Johnson); employees may own Johnson & Johnson stock/stock options. Alexa P. Kollmeier, Megan Miller, Yanli Wang, Shu Li, and May Shawi are employees of Janssen Research & Development, LLC (a subsidiary of Johnson & Johnson); employees may own Johnson & Johnson stock/stock options. Ya-Wen Yang is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson; employees may own Johnson & Johnson stock/stock options. Diamant Thaҫi has received honoraria for participation on advisory boards, as a speaker, and for consultancy from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Galderma, Janssen, Kyowa Hakko Kirin, La Roche-Posay, LEO Pharma, Merck Sharp & Dohme, Morphosys, New Bridge, Novartis, Pfizer, Regeneron Pharmaceuticals, Sandoz-Hexal, Sanofi, Sun Pharma, and UCB, and has received research grants from LEO Pharma and Novartis. Proton Rahman discloses consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB; travel support from Janssen; and grant/research support from Janssen and Novartis.
Figures

References
-
- Lebwohl M, Langley RG, Paul C, Puíg L, Reich K, van de Kerkhof P, et al. Evolution of patient perceptions of psoriatic disease: results from the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey. Dermatol Ther (Heidelb). 2022;12:61–78. doi: 10.1007/s13555-021-00635-4. - DOI - PMC - PubMed
-
- Samartín-Ucha M, Pego-Reigosa JM, Álvarez-Payero M, Martin-Vila A, Pineiro-Corrales G, Rodriguez-Rodriguez M, et al. Medication persistence on biological therapies prescribed for the treatment of chronic inflammatory arthropathies: a real-world data study. Eur J Hosp Pharm. 2021;28(Suppl 2):e47–50. doi: 10.1136/ejhpharm-2019-002133. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

