Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial
- PMID: 37906724
- PMCID: PMC10824373
- DOI: 10.1200/JCO.22.02867
Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial
Abstract
Purpose: In patients with peritoneal metastasis (PM) from gastric cancer (GC), chemotherapy is the treatment of choice. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are still being debated. This randomized, controlled, open-label, multicenter phase III trial (EudraCT 2006-006088-22; ClinicalTrials.gov identifier: NCT02158988) explored the impact on overall survival (OS) of HIPEC after CRS.
Patients and methods: Adult patients with GC and histologically proven PM were randomly assigned (1:1) to perioperative chemotherapy and CRS alone (CRS-A) or CRS plus HIPEC (CRS + H). HIPEC comprised mitomycin C 15 mg/m2 and cisplatin 75 mg/m2 in 5 L of saline perfused for 60 minutes at 42°C. The primary end point was OS; secondary endpoints included progression-free survival (PFS), other distant metastasis-free survival (MFS), and safety. Analyses followed the intention-to-treat principle.
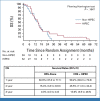
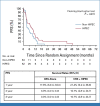
Results: Between March 2014 and June 2018, 105 patients were randomly assigned (53 patients to CRS-A and 52 patients to CRS + H). The trial stopped prematurely because of slow recruitment. In 55 patients, treatment stopped before CRS mainly due to disease progression/death. Median OS was the same for both groups (CRS + H, 14.9 [97.2% CI, 8.7 to 17.7] months v CRS-A, 14.9 [97.2% CI, 7.0 to 19.4] months; P = .1647). The PFS was 3.5 months (95% CI, 3.0 to 7.0) in the CRS-A group and 7.1 months (95% CI, 3.7 to 10.5; P = .047) in the CRS + H group. The CRS + H group showed better MFS (10.2 months [95% CI, 7.7 to 14.7] v CRS-A, 9.2 months [95% CI, 6.8 to 11.5]; P = .0286). The incidence of grade ≥3 adverse events (AEs) was similar between groups (CRS-A, 38.1% v CRS + H, 43.6%; P = .79).
Conclusion: This study showed no OS difference between CRS + H and CRS-A. PFS and MFS were significantly better in the CRS + H group, which needs further exploration. HIPEC did not increase AEs.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Bonnot PE, Piessen G, Kepenekian V, et al. : Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): A propensity score analysis. J Clin Oncol 37:2028-2040, 2019 - PubMed
-
- van Stein RM, Aalbers AGJ, Sonke GS, et al. : Hyperthermic intraperitoneal chemotherapy for ovarian and colorectal cancer: A review. JAMA Oncol 7:1231-1238, 2021 - PubMed
-
- Rau B, Loeffler M, Rau H-G, et al. : Perioperative chemotherapy and cytoreductive surgery with versus without HIPEC in gastric cancer with limited peritoneal metastases: A randomized phase III study (GASTRIPEC). J Clin Oncol 33, 2015. (suppl 15; abstr TPS4132)
-
- Pocock SJ: Clinical Trials—A Practical Approach. Chichester, New York, Brisbane, Toronto, Singapore, John Wiley & Sons, 1983, p 265
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

