First-in-human phase I/Ib study of NIZ985, a recombinant heterodimer of IL-15 and IL-15Rα, as a single agent and in combination with spartalizumab in patients with advanced and metastatic solid tumors
- PMID: 37907221
- PMCID: PMC10619015
- DOI: 10.1136/jitc-2023-007725
First-in-human phase I/Ib study of NIZ985, a recombinant heterodimer of IL-15 and IL-15Rα, as a single agent and in combination with spartalizumab in patients with advanced and metastatic solid tumors
Abstract
Background: Preclinically, interleukin-15 (IL-15) monotherapy promotes antitumor immune responses, which are enhanced when IL-15 is used in combination with immune checkpoint inhibitors (ICIs). This first-in-human study investigated NIZ985, a recombinant heterodimer comprising physiologically active IL-15 and IL-15 receptor α, as monotherapy and in combination with spartalizumab, an anti-programmed cell death protein-1 (anti-PD-1) monoclonal antibody, in patients with advanced solid tumors.
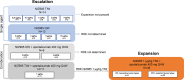
Methods: This phase I/Ib study had two dose-escalation arms: single-agent NIZ985 administered subcutaneously thrice weekly (TIW, 2 weeks on/2 weeks off) or once weekly (QW, 3 weeks on/1 week off), and NIZ985 TIW or QW administered subcutaneously plus spartalizumab (400 mg intravenously every 4 weeks (Q4W)). The dose-expansion phase investigated NIZ985 1 µg/kg TIW/spartalizumab 400 mg Q4W in patients with anti-PD-1-sensitive or anti-PD-1-resistant tumor types stratified according to approved indications. The primary objectives were the safety, tolerability, and the maximum tolerated doses (MTDs) and/or recommended dose for expansion (RDE) of NIZ985 for the dose-expansion phase.
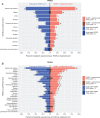
Results: As of February 17, 2020, 83 patients (median age: 63 years; range: 28-85) were treated in dose escalation (N=47; single-agent NIZ985: n=27; NIZ985/spartalizumab n=20) and dose expansion (N=36). No dose-limiting toxicities occurred nor was the MTD identified. The most common treatment-related adverse event (TRAE) was injection site reaction (primarily grades 1-2; single-agent NIZ985: 85% (23/27)); NIZ985/spartalizumab: 89% [50/56]). The most common grade 3-4 TRAE was decreased lymphocyte count (single-agent NIZ985: 7% [2/27]; NIZ985/spartalizumab: 5% [3/56]). The best overall response was stable disease in the single-agent arm (30% (8/27)) and partial response in the NIZ985/spartalizumab arm (5% [3/56]; melanoma, pancreatic cancer, gastric cancer). In dose expansion, the disease control rate was 45% (5/11) in the anti-PD-1-sensitive and 20% (5/25) in the anti-PD-1-resistant tumor type cohorts. Pharmacokinetic parameters were similar across arms. The transient increase in CD8+ T cell and natural killer cell proliferation and induction of several cytokines occurred in response to the single-agent and combination treatments.
Conclusions: NIZ985 was well tolerated in the single-agent and NIZ985/spartalizumab regimens. The RDE was established at 1 µg/kg TIW. Antitumor activity of the combination was observed against tumor types known to have a poor response to ICIs.
Trial registration number: NCT02452268.
Keywords: cytokines; immune checkpoint inhibitors; immunotherapy.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RL received research funding and travel expenses from Bristol Myers Squibb, and non-financial support from Clinigen and Celldex outside of the current work. DGM received research support to the institution from Novartis for the conduct of the study; consulting and research funding from Madison Vaccines; and research support to the University of Wisconsin from BMS, Merck, Harpoon and Janssen, unrelated to the current work. AW-G received fees for consulting or advisory role from BMS, Ipsen, Jacobio, Merrimack, Newlink, Pfizer and Rupugene, and is a full-time employee for Jacobio (unrelated to the current work). SG received support from Novartis for the conduct of the study, other support from Bristol Myers Squibb, Rexahn, Incyte, LSK, Five Prime, Mirati, QED, Debiopharm, Merck, Pfizer, AstraZeneca, Medimmune, Clovis and Seattle Genetics, and declares ownership of stock in Salarius Pharmaceuticals by the spouse. RW received consulting or advisory role fees from Puma Biotechnology, Pfizer, Roche Diagnostics, Seagen and Celcuity, fees from Celcuity for participating in the Scientific Steering committee and from Celcuity and Seagen for being an advisory board member, research funding from Acerta Pharma, and travel expenses from Puma Biotechnology, Pfizer and Roche Diagnostics. MC is an employee of IQVIA with a full-service provider role at Novartis. NH, JBL, LHL, JAO’K and NL are employees and stockholders of Novartis. GNP is a co-inventor on the heterodimeric IL-15 patents owned by the US government and managed by the National Institutes of Health (NIH), licensed to Novartis. GNP received research support from Novartis under the Collaborative Research Agreements (CRADAs) with the NIH during the period of this study. JAT received grants for consulting from Alpine Biosciences; research support to the University of Washington from Novartis during the conduct of the study; research support to the University of Washington from Pfizer, Five Prime, Merck, Trillium, Incyte and Xencor; and fees for consulting from Regeneron, Aveo, Neoleukin, Seattle Genetics, BJ Biosciences and Calithera. All authors received medical writing support from Novartis (non-financial). KC declares no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
