Cryo-EM structure of human O-GlcNAcylation enzyme pair OGT-OGA complex
- PMID: 37907462
- PMCID: PMC10618255
- DOI: 10.1038/s41467-023-42427-8
Cryo-EM structure of human O-GlcNAcylation enzyme pair OGT-OGA complex
Abstract
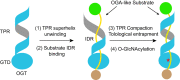
O-GlcNAcylation is a conserved post-translational modification that attaches N-acetyl glucosamine (GlcNAc) to myriad cellular proteins. In response to nutritional and hormonal signals, O-GlcNAcylation regulates diverse cellular processes by modulating the stability, structure, and function of target proteins. Dysregulation of O-GlcNAcylation has been implicated in the pathogenesis of cancer, diabetes, and neurodegeneration. A single pair of enzymes, the O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), catalyzes the addition and removal of O-GlcNAc on over 3,000 proteins in the human proteome. However, how OGT selects its native substrates and maintains the homeostatic control of O-GlcNAcylation of so many substrates against OGA is not fully understood. Here, we present the cryo-electron microscopy (cryo-EM) structures of human OGT and the OGT-OGA complex. Our studies reveal that OGT forms a functionally important scissor-shaped dimer. Within the OGT-OGA complex structure, a long flexible OGA segment occupies the extended substrate-binding groove of OGT and positions a serine for O-GlcNAcylation, thus preventing OGT from modifying other substrates. Conversely, OGT disrupts the functional dimerization of OGA and occludes its active site, resulting in the blocking of access by other substrates. This mutual inhibition between OGT and OGA may limit the futile O-GlcNAcylation cycles and help to maintain O-GlcNAc homeostasis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis.Int J Mol Sci. 2021 Mar 27;22(7):3463. doi: 10.3390/ijms22073463. Int J Mol Sci. 2021. PMID: 33801653 Free PMC article.
-
Elucidating the protein substrate recognition of O-GlcNAc transferase (OGT) toward O-GlcNAcase (OGA) using a GlcNAc electrophilic probe.Int J Biol Macromol. 2021 Feb 1;169:51-59. doi: 10.1016/j.ijbiomac.2020.12.078. Epub 2020 Dec 18. Int J Biol Macromol. 2021. PMID: 33333092 Free PMC article.
-
Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer.J Biol Chem. 2018 Sep 7;293(36):13989-14000. doi: 10.1074/jbc.RA118.004709. Epub 2018 Jul 23. J Biol Chem. 2018. PMID: 30037904 Free PMC article.
-
Regulating the Regulators: Mechanisms of Substrate Selection of the O-GlcNAc Cycling Enzymes OGT and OGA.Glycobiology. 2021 Aug 7;31(7):724-733. doi: 10.1093/glycob/cwab005. Glycobiology. 2021. PMID: 33498085 Free PMC article. Review.
-
[Protein O-GlcNAcylation and regulation of cell signalling: involvement in pathophysiology].Biol Aujourdhui. 2014;208(2):109-17. doi: 10.1051/jbio/2014015. Epub 2014 Sep 8. Biol Aujourdhui. 2014. PMID: 25190571 Review. French.
Cited by
-
Dissecting OGT's TPR domain to identify determinants of cellular function.Proc Natl Acad Sci U S A. 2024 May 28;121(22):e2401729121. doi: 10.1073/pnas.2401729121. Epub 2024 May 20. Proc Natl Acad Sci U S A. 2024. PMID: 38768345 Free PMC article.
-
Photoactivatable O-GlcNAc Transferase Library Enables Covalent Chemical Capture of Solvent-Exposed TPR Domain Interactions.Chembiochem. 2025 Jan 2;26(1):e202400709. doi: 10.1002/cbic.202400709. Epub 2024 Nov 25. Chembiochem. 2025. PMID: 39541256
-
The non-catalytic domains of O-GlcNAc cycling enzymes present new opportunities for function-specific control.Curr Opin Chem Biol. 2024 Aug;81:102476. doi: 10.1016/j.cbpa.2024.102476. Epub 2024 Jun 10. Curr Opin Chem Biol. 2024. PMID: 38861851 Free PMC article. Review.
-
Covalent Proximity Inducers.Chem Rev. 2025 Jan 8;125(1):326-368. doi: 10.1021/acs.chemrev.4c00570. Epub 2024 Dec 18. Chem Rev. 2025. PMID: 39692621 Free PMC article. Review.
-
O-GlcNAcylation promotes angiogenic transdifferentiation to reverse vascular ischemia.Nat Cardiovasc Res. 2025 Jul;4(7):904-920. doi: 10.1038/s44161-025-00673-7. Epub 2025 Jul 4. Nat Cardiovasc Res. 2025. PMID: 40615582
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

