A two-arm analysis of the immune response to heterologous boosting of inactivated SARS-CoV-2 vaccines
- PMID: 37907584
- PMCID: PMC10618206
- DOI: 10.1038/s41598-023-46053-8
A two-arm analysis of the immune response to heterologous boosting of inactivated SARS-CoV-2 vaccines
Abstract
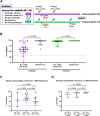
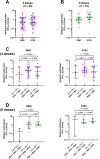
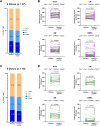
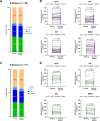
Several vaccine programs were introduced during the COVID-19 pandemic, which included inactivated virus, DNA viral vectors and mRNA vaccines. Booster programs are recommended, especially for those in high-risk groups. However, many of these booster programs involve heterologous vaccines. This study enrolled volunteers who first received two full-dose CoronaVac vaccinations before receiving heterologous boosters with DNA- and/or mRNA-vaccines for an additional 2 doses (n = 40) or an additional 3 doses (n = 16). Our results showed no difference in side effects, neutralizing antibodies, or T-cell responses for any of the heterologous vaccination programs. However, the neutralizing capacity and IFN-γ responses against the Omicron variant in volunteers who received 4 or 5 doses were improved. Polarization of peripheral memory T cells after stimulation in all booster groups with Omicron peptide showed an increased trend of naïve and central memory phenotypes of both CD4+ and CD8+ T cells, suggesting that exposure to Omicron antigens will drive T cells into a lymphoid resident T cell phenotype. Our data support a continuous vaccination program to maximize the effectiveness of immunity, especially in people at high risk. Furthermore, the number of boosting doses is important for maintaining immunity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Wu N, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023;11:439–452. doi: 10.1016/S2213-2600(23)00015-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

