Metabolic pathway analysis using stable isotopes in patients with cancer
- PMID: 37907620
- PMCID: PMC11161207
- DOI: 10.1038/s41568-023-00632-z
Metabolic pathway analysis using stable isotopes in patients with cancer
Abstract
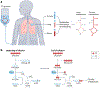
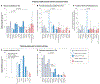
Metabolic reprogramming is central to malignant transformation and cancer cell growth. How tumours use nutrients and the relative rates of reprogrammed pathways are areas of intense investigation. Tumour metabolism is determined by a complex and incompletely defined combination of factors intrinsic and extrinsic to cancer cells. This complexity increases the value of assessing cancer metabolism in disease-relevant microenvironments, including in patients with cancer. Stable-isotope tracing is an informative, versatile method for probing tumour metabolism in vivo. It has been used extensively in preclinical models of cancer and, with increasing frequency, in patients with cancer. In this Review, we describe approaches for using in vivo isotope tracing to define fuel preferences and pathway engagement in tumours, along with some of the principles that have emerged from this work. Stable-isotope infusions reported so far have revealed that in humans, tumours use a diverse set of nutrients to supply central metabolic pathways, including the tricarboxylic acid cycle and amino acid synthesis. Emerging data suggest that some activities detected by stable-isotope tracing correlate with poor clinical outcomes and may drive cancer progression. We also discuss current challenges in isotope tracing, including comparisons of in vivo and in vitro models, and opportunities for future discovery in tumour metabolism.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
R.J.D. is a founder and adviser for Atavistik Bioscience and an adviser for Agios Pharmaceuticals, Vida Ventures and Droia Ventures. The other authors declare no competing interests.
Figures



References
-
- Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

