Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways
- PMID: 37907930
- PMCID: PMC10617163
- DOI: 10.1186/s12967-023-04648-9
Lycium barbarum glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways
Abstract
Background: Lycium barbarum polysaccharide (LBP) is an active ingredient extracted from Lycium barbarum that inhibits neuroinflammation, and Lycium barbarum glycopeptide (LbGp) is a glycoprotein with immunological activity that was purified and isolated from LBP. Previous studies have shown that LbGp can regulate the immune microenvironment, but its specific mechanism of action remains unclear.
Aims: In this study, we aimed to explore the mechanism of action of LbGp in the treatment of spinal cord injury through metabolomics and molecular experiments.
Methods: SD male rats were randomly assigned to three experimental groups, and after establishing the spinal cord hemisection model, LbGp was administered orally. Spinal cord tissue was sampled on the seventh day after surgery for molecular and metabolomic experiments. In vitro, LbGp was administered to mimic the inflammatory microenvironment by activating microglia, and its mechanism of action in suppressing neuroinflammation was further elaborated using metabolomics and molecular biology techniques such as western blotting and q-PCR.
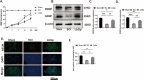
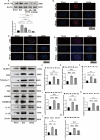
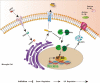
Results: In vivo and in vitro experiments found that LbGp can improve the inflammatory microenvironment by inhibiting the NF-kB and pyroptosis pathways. Furthermore, LbGp induced the secretion of docosahexaenoic acid (DHA) by microglia, and DHA inhibited neuroinflammation through the MAPK/NF-κB and pyroptosis pathways.
Conclusions: In summary, we hypothesize that LbGp improves the inflammatory microenvironment by regulating the secretion of DHA by microglia and thereby inhibiting the MAPK/NF-κB and pyroptosis pathways and promoting nerve repair and motor function recovery. This study provides a new direction for the treatment of spinal cord injury and elucidates the potential mechanism of action of LbGp.
Keywords: Docosahexaenoic acid; Lycium barbarum glycopeptide; Neuroinflammation; Spinal cord injury.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

