Nitric oxide and thiols: Chemical biology, signalling paradigms and vascular therapeutic potential
- PMID: 37908126
- PMCID: PMC11058123
- DOI: 10.1111/bph.16274
Nitric oxide and thiols: Chemical biology, signalling paradigms and vascular therapeutic potential
Abstract
Nitric oxide (• NO) interactions with biological thiols play crucial, but incompletely determined, roles in vascular signalling and other biological processes. Here, we highlight two recently proposed signalling paradigms: (1) the formation of a vasodilating labile nitrosyl ferrous haem (NO-ferrohaem) facilitated by thiols via thiyl radical generation and (2) polysulfides/persulfides and their interaction with • NO. We also describe the specific (bio)chemical routes in which • NO and thiols react to form S-nitrosothiols, a broad class of small molecules, and protein post-translational modifications that can influence protein function through catalytic site or allosteric structural changes. S-Nitrosothiol formation depends upon cellular conditions, but critically, an appropriate oxidant for either the thiol (yielding a thiyl radical) or • NO (yielding a nitrosonium [NO+ ]-donating species) is required. We examine the roles of these collective • NO/thiol species in vascular signalling and their cardiovascular therapeutic potential.
Keywords: NO-ferrohaem; S-nitrosothiols; labile haem; nitric oxide (•NO); nitrosation; nitrosylation; persulfides; polysulfides; thiyl radicals; vascular and cardiovascular disease.
© 2023 British Pharmacological Society.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
MRD and AWD are co-inventors of a provisional patent application filed at the University of Pittsburgh (Application No. 63/420030) related to the creation and use of NO–ferrohaem in biomedical applications.
Figures

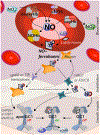
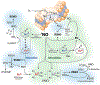
References
-
- Akaike T, Ida T, Wei F-Y, Nishida M, Kumagai Y, Alam MM, Ihara H, Sawa T, Matsunaga T, Kasamatsu S, Nishimura A, Morita M, Tomizawa K, Nishimura A, Watanabe S, Inaba K, Shima H, Tanuma N, Jung M, … Motohashi H (2017). Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nature Communications, 8, 1177. 10.1038/s41467-017-01311-y - DOI - PMC - PubMed
-
- Alexander SPH, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Davies JA, Boison D, Burns KE, Dessauer C, Gertsch J, Helsby NA, Izzo AA, Koesling D, … Wong SS (2021). The Concise Guide to PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178, S313–S411. - PubMed
-
- Alexander SPH, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Southan C, Buneman OP, Cidlowski JA, Christopoulos A, Davenport AP, Fabbro D, Spedding M, Striessnig J, Davies JA, Ahlers-Dannen KE, … Zolghadri Y (2021). The Concise Guide to PHARMACOLOGY 2021/22: Introduction and other protein targets. British Journal of Pharmacology, 178, S1–S26. - PMC - PubMed
-
- Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, & O’Connor CM (2020). Vericiguat in patients with heart failure and reduced ejection fraction. The New England Journal of Medicine, 382, 1883–1893. 10.1056/NEJMoa1915928 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

