Enhanced expression of natural cytotoxicity receptors on cytokine-induced memory-like natural killer cells correlates with effector function
- PMID: 37908353
- PMCID: PMC10613704
- DOI: 10.3389/fimmu.2023.1256404
Enhanced expression of natural cytotoxicity receptors on cytokine-induced memory-like natural killer cells correlates with effector function
Abstract
Introduction: Natural killer (NK) cells are a key component of the innate immune system, involved in defending the host against virus-infected cells and tumor immunosurveillance. Under in vitro culture conditions, IL-12/15/18 can induce a memory-like phenotype in NK cells. These cytokine-induced memory-like (CIML) NK cells possess desirable characteristics for immunotherapies, including a longer lifespan and increased cytotoxicity.
Methods: In this study, NK cells were isolated from peripheral blood of healthy donors and stimulated with IL-12/15/18 to induce a memory-like phenotype or with IL-15 alone as a control. After seven days of culture, multiparametric flow cytometry analysis was performed to evaluate the phenotypic and functional profiles of CIML and control NK cells.
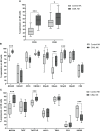
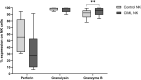
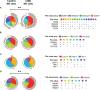
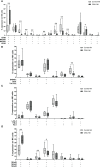
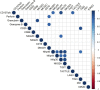
Results: Our results showed a significantly higher expression of CD25, CD69, NKG2D, NKp30, NKp44, NKp46, TACTILE, and Granzyme B in CIML NK cells compared to control NK cells. In contrast, KIR2D expression was significantly lower in CIML NK cells than in control NK cells. Moreover, functional experiments demonstrated that CIML NK cells displayed enhanced degranulation capacity and increased intracellular IFN-γ production against the target cell line K562. Interestingly, the degranulation capacity of CIML NK cells was positively correlated with the expression of the activating receptors NKp46 and NKp30, as well as with the inhibitory receptor TACTILE.
Discussion: In conclusion, this study provides a deep phenotypic characterization of in vitro-expanded CIML NK cells. Moreover, the correlations found between NK cell receptors and degranulation capacity of CIML NK cells allowed the identification of several biomarkers that could be useful in clinical settings.
Keywords: NK cells; NKG2D; cancer immunotherapy; cytokine-induced memory-like NK cells; degranulation capacity; memory-like; natural cytotoxicity receptors.
Copyright © 2023 Carreira-Santos, López-Sejas, González-Sánchez, Sánchez-Hernández, Pera, Hassouneh, Durán, Solana, Casado and Tarazona.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
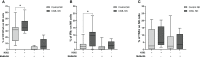
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

