Safety and immunogenicity of BK-SE36/CpG malaria vaccine in healthy Burkinabe adults and children: a phase 1b randomised, controlled, double-blinded, age de-escalation trial
- PMID: 37908361
- PMCID: PMC10613650
- DOI: 10.3389/fimmu.2023.1267372
Safety and immunogenicity of BK-SE36/CpG malaria vaccine in healthy Burkinabe adults and children: a phase 1b randomised, controlled, double-blinded, age de-escalation trial
Abstract
Background: BK-SE36/CpG is a recombinant blood-stage malaria vaccine candidate based on the N-terminal Plasmodium falciparum serine repeat antigen5 (SE36), adsorbed to aluminium hydroxide gel and reconstituted, prior to administration, with synthetic oligodeoxynucleotides bearing CpG motifs. In healthy Japanese adult males, BK-SE36/CpG was well tolerated. This study assessed its safety and immunogenicity in healthy malaria-exposed African adults and children.
Methods: A double-blind, randomised, controlled, age de-escalating clinical trial was conducted in an urban area of Ouagadougou, Burkina Faso. Healthy participants (n=135) aged 21-45 years (Cohort 1), 5-10 years (Cohort 2) and 12-24 months (Cohort 3) were randomised to receive three vaccine doses (Day 0, 28 and 112) of BK-SE36/CpG or rabies vaccine by intramuscular injection.
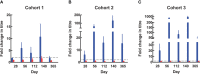
Results: One hundred thirty-four of 135 (99.2%) subjects received all three scheduled vaccine doses. Vaccinations were well tolerated with no related Grade 3 (severe) adverse events (AEs). Pain/limitation of limb movement, headache in adults and fever in younger children (all mild to moderate in intensity) were the most frequently observed local and systemic AEs. Eighty-three of BK-SE36/CpG (91%) recipients and 37 of control subjects (84%) had Grade 1/2 events within 28 days post vaccination. Events considered by the investigator to be vaccine related were experienced by 38% and 14% of subjects in BK-SE36/CpG and control arms, respectively. Throughout the trial, six Grade 3 events (in 4 subjects), not related to vaccination, were recorded in the BK-SE36/CpG arm: 5 events (in 3 subjects) within 28 days of vaccination. All serious adverse events (SAEs) (n=5) were due to severe malaria (52-226 days post vaccination) and not related to vaccination. In all cohorts, BK-SE36/CpG arm had higher antibody titres after Dose 3 than after Dose 2. Younger cohorts had stronger immune responses (12-24-month-old > 5-10 years-old > 21-45 years-old). Sera predominantly reacted to peptides that lie in intrinsically unstructured regions of SE36. In the control arm, there were no marked fold changes in antibody titres and participants' sera reacted poorly to all peptides spanning SE36.
Conclusion: BK-SE36/CpG was well-tolerated and immunogenic. These results pave the way for further proof-of-concept studies to demonstrate vaccine efficacy.
Clinical trial registration: https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=1921, PACTR201701001921166.
Keywords: BK-SE36/CpG; Plasmodium falciparum; SERA5; immunogenicity; malaria vaccine; safety; serine repeat antigen.
Copyright © 2023 Ouédraogo, Bougouma, Palacpac, Houard, Nebie, Sawadogo, Berges, Soulama, Diarra, Hien, Ouedraogo, Konaté, Kouanda, Myoui, Ezoe, Ishii, Sato, D’Alessio, Leroy, Tiono, Cousens, Horii and Sirima.
Conflict of interest statement
TH is the inventor of BK-SE36; TH and KI are inventors of BK-SE36/CpG. NP and TH are supported by a research fund from Nobelpharma Co., Ltd NPC, the clinical trial sponsor. TS is an employee and SE is medical adviser of NPC. These involvements did not influence the design of the study, the collection, analyses, access to and interpretation of data, and the writing of this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- World Health Organization . World Malaria Report. (2022). (Geneva: WHO; ). Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria... (Accessed June 9, 2023).
-
- World Health Organization . WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk (2021) (Geneva: WHO; ). Available at: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-m... (Accessed June 9, 2023).
-
- World Health Organization . Global Technical Strategy for Malaria 2016-2030, 2021 Update (2021). Available at: https://www.who.int/publications/i/item/9789240031357 (Accessed June 9, 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

