Real-world characteristics of "super-responders" to mepolizumab and benralizumab in severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis
- PMID: 37908397
- PMCID: PMC10613971
- DOI: 10.1183/23120541.00419-2023
Real-world characteristics of "super-responders" to mepolizumab and benralizumab in severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis
Abstract
Background: The current definition of severe eosinophilic asthma (SEA) super-responders to biologic treatment does not include patients with other eosinophil-based comorbidities. Although eosinophilic granulomatosis with polyangiitis (EGPA) is frequently associated with SEA, we lack data on a possible super-response to biologic treatments in patients suffering from these two diseases. We aim to assess super-responder features in real-life patients with SEA and EGPA treated with mepolizumab and benralizumab.
Methods: We enrolled 39 patients with SEA and EGPA eligible for treatment with mepolizumab or benralizumab. Super-responder assessment was performed considering oral corticosteroid (OCS) cessation, lack of exacerbations, forced expiratory volume in 1 s and Asthma Control Test (ACT) improvement.
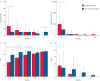
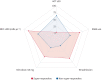
Results: Super-responders showed worse clinical baseline characteristics than non-super-responder patients, with a greater improvement in severe asthma exacerbations, OCS dose reduction and ACT score increase. Definition of super-responders was consistent only considering a 12-month course of monoclonal antibody, lacking sensitivity in earlier evaluations.
Conclusion: Mepolizumab and benralizumab are safe and effective in patients with EGPA and SEA, since a consistent proportion of patients show a super-response after 12 months of treatment. Further studies will address specific criteria for super-responder assessment in these patients.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: A. Portacci reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Chiesi and Sanofi. Conflict of interest: N. Crimi reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline and Sanofi. Conflict of interest: A. Benfante reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline and Sanofi. Conflict of interest: M. Triggiani reports consulting fees from AstraZeneca, GlaxoSmithKline and Novartis. Conflict of interest: G. Scioscia reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline and Sanofi. Conflict of interest: A. Detoraki reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Sanofi, Novartis and Lofarma. Conflict of interest: G. Valenti reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GlaxoSmithKline and Sanofi. Conflict of interest: N. Scichilone reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Chiesi and Sanofi. Conflict of interest: G. Pelaia reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Chiesi, Sanofi, Guidotti, Menarini and Novartis. Conflict of interest: C. Crimi reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Astrazeneca, GlaxoSmithKline, Sanofi, Menarini, ResMed and Fisher&Paykel. Conflict of interest: G.E. Carlagnano reports grants or contracts from AstraZeneca, Chiesi, GlaxoSmithKline, Sanofi and Grifols; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline and Sanofi; and support for attending meetings and/or travel from Astrazeneca, Menarini and Chiesi.
Figures
References
LinkOut - more resources
Full Text Sources
