Clinical and Health Economic Evaluation of a Novel Device for Fecal Management in Bedridden Patients
- PMID: 37908427
- PMCID: PMC10613872
- DOI: 10.5005/jp-journals-10071-24544
Clinical and Health Economic Evaluation of a Novel Device for Fecal Management in Bedridden Patients
Abstract
Purpose: To evaluate the clinical effectiveness and health economic benefits of a novel indwelling lattice-based device for fecal management in bedridden patients.
Materials and methods: This nonrandomized, two-arm study included 70 bedridden patients (≥18 years exhibiting liquid stool) referred from the ICU of surgery and medicine units of a 2000-bed tertiary care referral hospital, assigned to the intervention and control groups. About 35 patients were eligible to be included in the intervention group while 35 patients with contraindications to the intervention device were included in the usual care control group. Assessments were made before and every 24 hours during the study, and all patients were closely monitored for development of incontinence-associated dermatitis (IAD) and hospital-acquired pressure injury.
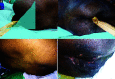
Results: The test device was successfully deployed on the first attempt and effectively diverted fecal matter in all 35 patients, with no adverse events. In the control group, 83% of the patients developed IAD, which resulted in prolonged hospitalization and increased expenses. Overall, the control group (with adult diapers) required greater time, resources, and efforts for fecal management and resulted in increased patient morbidity.
Conclusion: The patient management time, resource consumption, overall cost of hospital admission, and the complication rates are significantly lower with the use of the novel lattice-based device than with the use of adult diapers for fecal management.
How to cite this article: Sheth H, Rao S, Karthik V. Clinical and Health Economic Evaluation of a Novel Device for Fecal Management in Bedridden Patients. Indian J Crit Care Med 2023;27(10):759-765.
Keywords: Balloon catheter; Critical care; Dermatitis; Diarrhea; FMS; Fecal incontinence; Fecal management; Hospital-acquired pressure injury (HAPI); Incontinence-associated dermatitis; Pressure ulcer.
Copyright © 2023; The Author(s).
Conflict of interest statement
Source of support: The test devices used in the study are provided by Consure Medical, New Delhi, India Conflict of interest: None
Figures

References
-
- Padula WV, Makic MBF, Wald HL, Campbell JD, Nair KV, Mishra MK, et al. Hospital-acquired pressure ulcers at academic medical centers in the united states, 2008–2012: tracking changes since the cms nonpayment policy. Jt Comm J Qual Patient Saf. 2015;41(6):257–263. doi: 10.1016/s1553-7250(15)41035-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
