Inhalation of acidic nanoparticles prevents doxorubicin cardiotoxicity through improvement of lysosomal function
- PMID: 37908733
- PMCID: PMC10614672
- DOI: 10.7150/thno.86310
Inhalation of acidic nanoparticles prevents doxorubicin cardiotoxicity through improvement of lysosomal function
Abstract
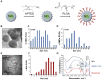
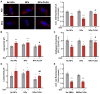
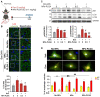
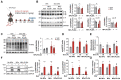
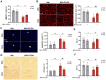
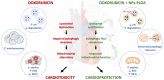
Doxorubicin (Dox) is an effective anticancer molecule, but its clinical efficacy is limited by strong cardiotoxic side effects. Lysosomal dysfunction has recently been proposed as a new mechanism of Dox-induced cardiomyopathy. However, to date, there is a paucity of therapeutic approaches capable of restoring lysosomal acidification and function in the heart. Methods: We designed novel poly(lactic-co-glycolic acid) (PLGA)-grafted silica nanoparticles (NPs) and investigated their therapeutic potential in the primary prevention of Dox cardiotoxicity in cardiomyocytes and mice. Results: We showed that NPs-PLGA internalized rapidly in cardiomyocytes and accumulated inside the lysosomes. Mechanistically, NPs-PLGA restored lysosomal acidification in the presence of doxorubicin or bafilomycin A1, thereby improving lysosomal function and autophagic flux. Importantly, NPs-PLGA mitigated Dox-related mitochondrial dysfunction and oxidative stress, two main mechanisms of cardiotoxicity. In vivo, inhalation of NPs-PLGA led to effective and rapid targeting of the myocardium, which prevented Dox-induced adverse remodeling and cardiac dysfunction in mice. Conclusion: Our findings demonstrate a pivotal role for lysosomal dysfunction in Dox-induced cardiomyopathy and highlight for the first time that pulmonary-driven NPs-PLGA administration is a promising strategy against anthracycline cardiotoxicity.
Keywords: autophagy; cardiotoxicity; doxorubicin; lysosomes; nanoparticles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JAP, Saffi J. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90:2063–76. - PubMed
-
- Colombo A, Cipolla C, Beggiato M, Cardinale D. Cardiac toxicity of anticancer agents. Curr Cardiol Rep. 2013;15:362. - PubMed
-
- Li M, Sala V, De Santis MC, Cimino J, Cappello P, Pianca N. et al. Phosphoinositide 3-Kinase Gamma Inhibition Protects From Anthracycline Cardiotoxicity and Reduces Tumor Growth. Circulation. 2018;138:696–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

