Gut microbiota depletion aggravates bile acid-induced liver pathology in mice with a human-like bile acid composition
- PMID: 37910096
- PMCID: PMC10643054
- DOI: 10.1042/CS20230812
Gut microbiota depletion aggravates bile acid-induced liver pathology in mice with a human-like bile acid composition
Abstract
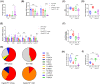
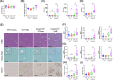
Cyp2c70-deficient mice have a human-like bile acid (BA) composition due to their inability to convert chenodeoxycholic acid (CDCA) into rodent-specific muricholic acids (MCAs). However, the hydrophobic BA composition in these animals is associated with liver pathology. Although Cyp2c70-ablation has been shown to alter gut microbiome composition, the impact of gut bacteria on liver pathology in Cyp2c70-/- mice remains to be established. Therefore, we treated young-adult male and female wild-type (WT) and Cyp2c70-/- mice with antibiotics (AB) with broad specificity to deplete the gut microbiota and assessed the consequences on BA metabolism and liver pathology. Female Cyp2c70-/- mice did not tolerate AB treatment, necessitating premature termination of the experiment. Male Cyp2c70-/- mice did tolerate AB but showed markedly augmented liver pathology after 6 weeks of treatment. Dramatic downregulation of hepatic Cyp8b1 expression (-99%) caused a reduction in the proportions of 12α-hydroxylated BAs in the circulating BA pools of AB-treated male Cyp2c70-/- mice. Interestingly, the resulting increased BA hydrophobicity strongly correlated with various indicators of liver pathology. Moreover, genetic inactivation of Cyp8b1 in livers of male Cyp2c70-/- mice increased liver pathology, while addition of ursodeoxycholic acid to the diet prevented weight loss and liver pathology in AB-treated female Cyp2c70-/- mice. In conclusion, depletion of gut microbiota in Cyp2c70-/- mice aggravates liver pathology at least in part by increasing the hydrophobicity of the circulating BA pool. These findings highlight that the potential implications of AB administration to cholestatic patients should be evaluated in a systematic manner.
Keywords: Cyp2c70; bile acids; gut microbiota; hepatobiliary disease; hydrophobicity; liver fibrosis.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

