A tutorial on asymmetric electrocatalysis
- PMID: 37910160
- PMCID: PMC10842033
- DOI: 10.1039/d3cs00511a
A tutorial on asymmetric electrocatalysis
Erratum in
-
Correction: A tutorial on asymmetric electrocatalysis.Chem Soc Rev. 2024 Jan 2;53(1):545. doi: 10.1039/d3cs90096g. Chem Soc Rev. 2024. PMID: 38050457 Free PMC article.
Abstract
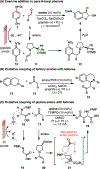
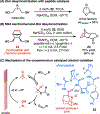
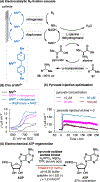
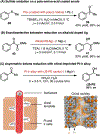
Electrochemistry has emerged as a powerful means to enable redox transformations in modern chemical synthesis. This tutorial review delves into the unique advantages of electrochemistry in the context of asymmetric catalysis. While electrochemistry has historically been used as a green and mild alternative for established enantioselective transformations, in recent years asymmetric electrocatalysis has been increasingly employed in the discovery of novel asymmetric methodologies based on reaction mechanisms unique to electrochemistry. This tutorial review first provides a brief tutorial introduction to electrosynthesis, then explores case studies on homogenous small molecule asymmetric electrocatalysis. Each case study serves to highlight a key advance in the field, starting with the historic electrification of known asymmetric transformations and culminating with modern methods relying on unique electrochemical mechanistic sequences. Finally, we highlight case studies in the emerging reasearch areas at the interface of asymmetric electrocatalysis with biocatalysis and heterogeneous catalysis.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures






References
-
- Jacobsen EN, Pfaltz A and Yamamoto H, Comprehensive Asymmetric Catalysis, Springer Berlin Heidelberg, 2004.
-
- Gnas Y and Glorius F, Synthesis, 2006, 1899–1930.
-
- Teng Y, Gu C, Chen Z, Jiang H, Xiong Y, Liu D and Xiao D, Chirality, 2022, 34, 1094–1119. - PubMed
-
- Knowles WS, Adv. Synth. Catal, 2003, 345, 3–13.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

