Intrathecal delivery of nanoparticle PARP inhibitor to the cerebrospinal fluid for the treatment of metastatic medulloblastoma
- PMID: 37910601
- PMCID: PMC11078331
- DOI: 10.1126/scitranslmed.adi1617
Intrathecal delivery of nanoparticle PARP inhibitor to the cerebrospinal fluid for the treatment of metastatic medulloblastoma
Abstract
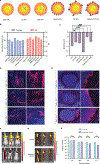
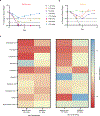
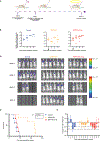
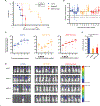
The morbidity associated with pediatric medulloblastoma, in particular in patients who develop leptomeningeal metastases, remains high in the absence of effective therapies. Administration of substances directly into the cerebrospinal fluid (CSF) is one approach to circumvent the blood-brain barrier and focus delivery of drugs to the site of tumor. However, high rates of CSF turnover prevent adequate drug accumulation and lead to rapid systemic clearance and toxicity. Here, we show that PLA-HPG nanoparticles, made with a single-emulsion, solvent evaporation process, can encapsulate talazoparib, a PARP inhibitor (BMN-673). These degradable polymer nanoparticles improve the therapeutic index when delivered intrathecally and lead to sustained drug retention in the tumor as measured with PET imaging and fluorescence microscopy. We demonstrate that administration of these particles into the CSF, alone or in combination with systemically administered temozolomide, is a highly effective therapy for tumor regression and prevention of leptomeningeal spread in xenograft mouse models of medulloblastoma. These results provide a rationale for harnessing nanoparticles for the delivery of drugs limited by brain penetration and therapeutic index and demonstrate important advantages in tolerability and efficacy for encapsulated drugs delivered locoregionally.
Conflict of interest statement
Competing interests
MK, RSB, and WMS are inventors on a patent application describing intrathecal administration of nanoparticles for treating cancer (Title: INTRATHECAL NANOPARTICLE DELIVERY FOR TREATMENT OF LEPTOMENINGEAL TUMORS, U.S. Patent Application No. 63/383,211). RSB and WMS are co-founders of B3 Therapeutics. WMS is a consultant to Xanadu Bio, B3 Therapeutics, Stradefy Biosciences, Johnson & Johnson, Celanese, Cranius, and CMC Pharma. RSB is a consultant to Cybrexa therapeutics, Alphina therapeutics, Modifi bio, Sage Biosciences (SAB), and Aprea (SAB). The rest of the authors declare that they have no competing interests.
Figures




References
-
- Rutkowski S, Cohen B, Finlay J, Luksch R, Ridola V, Valteau-Couanet D, Hara J, Garre ML, Grill J, Medulloblastoma in young children, Pediatr. Blood Cancer 54, 635–637 (2010). - PubMed
-
- Gilbertson RJ, Ellison DW, The origins of medulloblastoma subtypes, Annu. Rev. Pathol. 3, 341–365 (2008). - PubMed
-
- Kann BH, Park HSM, Lester-Coll NH, Yeboa DN, Benitez V, Bindra RS, Khan AJ, Marks AM, Roberts KB, Adjuvant Radiation Therapy Patterns and Survival Implications for Medulloblastoma in Young Children, Int. J. Radiat. Oncol 96, 1574–1581 (2016). - PubMed
-
- Koschmann C, Bloom K, Upadhyaya S, Geyer JR, Leary SES, Survival After Relapse of Medulloblastoma, J. Pediatr. Hematol. Oncol. 38, 269–273 (2016). - PubMed
-
- Zapotocky M, Mata-Mbemba D, Sumerauer D, Liby P, Lassaletta A, Zamecnik J, Krskova L, Kyncl M, Stary J, Laughlin S, Arnoldo A, Hawkins C, Tabori U, Taylor MD, Bouffet E, Raybaud C, Ramaswamy V, Differential patterns of metastatic dissemination across medulloblastoma subgroups, J. Neurosurg. Pediatr. 21, 145–152 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

