RANKL/RANK is required for cytokine-induced beta cell death; osteoprotegerin, a RANKL inhibitor, reverses rodent type 1 diabetes
- PMID: 37910614
- PMCID: PMC10619938
- DOI: 10.1126/sciadv.adf5238
RANKL/RANK is required for cytokine-induced beta cell death; osteoprotegerin, a RANKL inhibitor, reverses rodent type 1 diabetes
Abstract
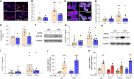
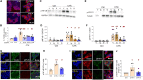
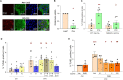
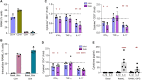
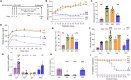
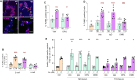
Treatment for type 1 diabetes (T1D) requires stimulation of functional β cell regeneration and survival under stress. Previously, we showed that inhibition of the RANKL/RANK [receptor activator of nuclear factor kappa Β (NF-κB) ligand] pathway, by osteoprotegerin and the anti-osteoporotic drug denosumab, induces rodent and human β cell proliferation. We demonstrate that the RANK pathway mediates cytokine-induced rodent and human β cell death through RANK-TRAF6 interaction and induction of NF-κB activation. Osteoprotegerin and denosumab protected β cells against this cytotoxicity. In human immune cells, osteoprotegerin and denosumab reduce proinflammatory cytokines in activated T-cells by inhibiting RANKL-induced activation of monocytes. In vivo, osteoprotegerin reversed recent-onset T1D in nonobese diabetic/Ltj mice, reduced insulitis, improved glucose homeostasis, and increased plasma insulin, β cell proliferation, and mass in these mice. Serum from T1D subjects induced human β cell death and dysfunction, but not α cell death. Osteoprotegerin and denosumab reduced T1D serum-induced β cell cytotoxicity and dysfunction. Inhibiting RANKL/RANK could have therapeutic potential.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

