Severe acute malnutrition promotes bacterial binding over proinflammatory cytokine secretion by circulating innate immune cells
- PMID: 37910623
- PMCID: PMC10619937
- DOI: 10.1126/sciadv.adh2284
Severe acute malnutrition promotes bacterial binding over proinflammatory cytokine secretion by circulating innate immune cells
Abstract
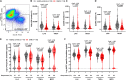
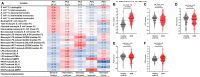
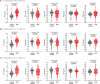
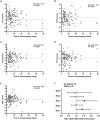
Children with severe acute malnutrition (SAM) have high infectious mortality and morbidity, implicating defects in their immune defenses. We hypothesized that circulating innate immune cells from children (0 to 59 months) hospitalized with SAM in Zambia and Zimbabwe (n = 141) have distinct capacity to respond to bacteria relative to adequately nourished healthy controls (n = 92). SAM inpatients had higher neutrophil and monocyte Escherichia coli binding capacity but lower monocyte activation and proinflammatory mediator secretion in response to lipopolysaccharide or heat-killed Salmonella typhimurium than controls. Among SAM cases, wasting severity was negatively associated with cytokine secretion, children with HIV had lower monocyte activation, and the youngest children released the least myeloperoxidase upon stimulation. Inpatient bacterial binding capacity and monocyte activation were associated with higher odds of persistent SAM at discharge, a risk factor for subsequent mortality. Thus, SAM shifts innate immune cell function, favoring bacterial containment over proinflammatory activation, which may contribute to health deficits after discharge.
Figures


References
-
- UNICEF, WHO, World Bank, Levels and Trends in Child Malnutrition: UNICEF/WHO/The World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2021 Edition (WHO, 2021).
-
- WHO, Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children (WHO, 2013). - PubMed
-
- G. WHO, Management of the Child with a Serious Infection or Severe Malnutrition: Guidelines for Care at the First-Referral Level in Developing Countries (WHO, 2000).
-
- Sharrow D., Hug L., You D., Alkema L., Black R., Cousens S., Croft T., Gaigbe-Togbe V., Gerland P., Guillot M., Hill K., Masquelier B., Mathers C., Pedersen J., Strong K. L., Suzuki E., Wakefield J., Walker N.; UN Inter-agency Group for Child Mortality Estimation and its Technical Advisory Group , Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: A systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health 10, e195–e206 (2022). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

