Cannabidiol as an Alternative Analgesic for Acute Dental Pain
- PMID: 37910667
- PMCID: PMC10900863
- DOI: 10.1177/00220345231200814
Cannabidiol as an Alternative Analgesic for Acute Dental Pain
Erratum in
-
Corrigendum to "Cannabidiol as an Alternative Analgesic for Acute Dental Pain".J Dent Res. 2024 Jul;103(7):767-768. doi: 10.1177/00220345241257653. Epub 2024 Jun 18. J Dent Res. 2024. PMID: 38900211 Free PMC article. No abstract available.
Abstract
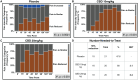
Odontogenic pain can be debilitating, and nonopioid analgesic options are limited. This randomized placebo-controlled clinical trial aimed to assess the effectiveness and safety of cannabidiol (CBD) as an analgesic for patients with emergency acute dental pain. Sixty-one patients with moderate to severe toothache were randomized into 3 groups: CBD10 (CBD 10 mg/kg), CBD20 (CBD 20 mg/kg), and placebo. We administered a single dose of respective oral solution and monitored the subjects for 3 h. The primary outcome measure was the numerical pain differences using a visual analog scale (VAS) from baseline within and among the groups. Secondary outcome measures included ordinal pain intensity differences, the onset of significant pain relief, maximum pain relief, changes in bite force within and among the groups, psychoactive effects, mood changes, and other adverse events. Both CBD groups resulted in significant VAS pain reduction compared to their baseline and the placebo group, with a maximum median VAS pain reduction of 73% from baseline pain at the 180-min time point (P < 0.05). CBD20 experienced a faster onset of significant pain relief than CBD10 (15 versus 30 min after drug administration), and both groups reached maximum pain relief at 180-min. Number needed to treat was 3.1 for CBD10 and 2.4 for CBD20. Intragroup comparisons showed a significant increase in bite forces in both CBD groups (P < 0.05) but not in the placebo group (P > 0.05). CBD20 resulted in a significant difference in mean percent bite force change in the 90- and 180-min time points compared to the placebo group (P < 0.05). Compared to placebo, sedation, diarrhea, and abdominal pain were significantly associated with the CBD groups (P < 0.05). There were no other significant psychoactive or mood change effects. This randomized trial provides the first clinical evidence that oral CBD can be an effective and safe analgesic for dental pain.
Keywords: analgesics; clinical trial; endodontics; non-narcotic; pain measurement; toothache.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Comment in
-
Cannabidiol for Toothache: Ups, Downs, and Regulatory Considerations.J Dent Res. 2024 Mar;103(3):225-226. doi: 10.1177/00220345231223691. Epub 2024 Feb 12. J Dent Res. 2024. PMID: 38347664 Free PMC article. No abstract available.
-
Letter to the Editor, "Cannabinoids and Acute Dental Pain".J Dent Res. 2024 Jul;103(7):765. doi: 10.1177/00220345241238688. Epub 2024 Jun 18. J Dent Res. 2024. PMID: 38900209 No abstract available.
References
-
- Alelyani AA, Azar PS, Khan AA, Chrepa V, Diogenes A. 2020. Quantitative assessment of mechanical allodynia and central sensitization in endodontic patients. J Endod. 46(12):1841–1848. - PubMed
-
- Bradford AC, Bradford WD. 2017. Medical marijuana laws may be associated with a decline in the number of prescriptions for Medicaid enrollees. Health Aff (Millwood). 36(5):945–951. - PubMed
-
- Bruno A, Tacconelli S, Patrignani P. 2014. Variability in the response to non-steroidal anti-inflammatory drugs: mechanisms and perspectives. Basic Clin Pharmacol Toxicol. 114(1):56–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

