Innate immunity: Looking beyond T-cells in radiation and immunotherapy combinations
- PMID: 37913654
- PMCID: PMC10637988
- DOI: 10.1016/j.neo.2023.100940
Innate immunity: Looking beyond T-cells in radiation and immunotherapy combinations
Abstract
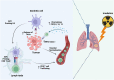
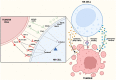
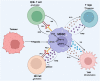
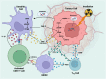
Radiation therapy is an established and effective anti-cancer treatment modality. Extensive pre-clinical experimentation has demonstrated that the pro-inflammatory properties of irradiation may be synergistic with checkpoint immunotherapy. Radiation induces double-stranded DNA breaks (dsDNA). Sensing of the dsDNA activates the cGAS/STING pathway, producing Type 1 interferons essential to recruiting antigen-presenting cells (APCs). Radiation promotes cytotoxic CD8 T-cell recruitment by releasing tumour-associated antigens captured and cross-presented by surveying antigen-presenting cells. Radiation-induced vascular normalisation may further promote T-cell trafficking and drug delivery. Radiation is also immunosuppressive. Recruitment of regulatory T cells (Tregs) and innate cells such as myeloid-derived suppressive cells (m-MDSCs) all counteract the immunostimulatory properties of radiation. Many innate immune cell types operate at the interface of the adaptive immune response. Innate immune cells, such as m-MDSCs, can exert their immunosuppressive effects by expressing immune checkpoints such as PD-L1, further highlighting the potential of combined radiation and checkpoint immunotherapy. Several early-phase clinical studies investigating the combination of radiation and immunotherapy have been disappointing. A greater appreciation of radiotherapy's impact on the innate immune system is essential to optimise radioimmunotherapy combinations. This review will summarise the impact of radiotherapy on crucial cells of the innate immune system and vital immunosuppressive cytokines.
Keywords: Immune system; Immunotherapy; Innate; Radiation; Radiotherapy.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest I can confirm that the above authors have no conflicts of interest. This manuscript has not received any financial assistance or funding nor been submitted to any other journals for review/publication.
Figures
References
-
- Dunn PL, North RJ. Selective radiation resistance of immunologically induced T cells as the basis for irradiation-induced T-cell-mediated regression of immunogenic tumor. J. Leukoc. Biol. 1991;49(4):388–396. - PubMed
-
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J. Immunol. 2002;169(7):3760–3770. - PubMed
-
- Sia JI. University of Melbourne; 2020. Study of the Immunomodulatory Effects of Radiation Therapy in Solid Cancers.
-
- Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

