Transplanting old organs promotes senescence in young recipients
- PMID: 37913871
- PMCID: PMC10922683
- DOI: 10.1016/j.ajt.2023.10.013
Transplanting old organs promotes senescence in young recipients
Abstract
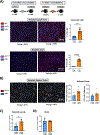
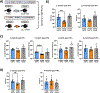
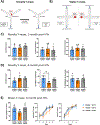
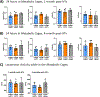
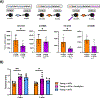
In clinical organ transplantation, donor and recipient ages may differ substantially. Old donor organs accumulate senescent cells that have the capacity to induce senescence in naïve cells. We hypothesized that the engraftment of old organs may induce senescence in younger recipients, promoting age-related pathologies. When performing isogeneic cardiac transplants between age-mismatched C57BL/6 old donor (18 months) mice and young and middle-aged C57BL/6 (3- or 12- month-old) recipients , we observed augmented frequencies of senescent cells in draining lymph nodes, adipose tissue, livers, and hindlimb muscles 30 days after transplantation. These observations went along with compromised physical performance and impaired spatial learning and memory abilities. Systemic levels of the senescence-associated secretory phenotype factors, including mitochondrial DNA (mt-DNA), were elevated in recipients. Of mechanistic relevance, injections of mt-DNA phenocopied effects of age-mismatched organ transplantation on accelerating aging. Single treatment of old donor animals with senolytics prior to transplantation attenuated mt-DNA release and improved physical capacities in young recipients. Collectively, we show that transplanting older organs induces senescence in transplant recipients, resulting in compromised physical and cognitive capacities. Depleting senescent cells with senolytics, in turn, represents a promising approach to improve outcomes of older organs.
Keywords: Aging; Senescence; age-related pathologies; cellular senescence; ischemia-reperfusion injury; organ allocation; senescence-associated secretory phenotype; senolytics.
Copyright © 2023 American Society of Transplantation & American Society of Transplant Surgeons. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests
J.L.K., T.T., and S.G.T. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic and Brigham and Women’s Hospital. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. No conflicts of interest, financial or otherwise, are declared by the other authors.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Stefan G. Tullius has patent Methods And Materials For Improving Transplant Outcomes licensed to Stefan G. Tullius.
James L. Kirkland has patent Methods And Materials For Improving Transplant Outcomes licensed to Stefan G. Tullius.
Tamar Tchkonia has patent Methods And Materials For Improving Transplant Outcomes licensed to Stefan G. Tullius.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

