Nationwide Retrospective Analysis of Combinations of Advanced Therapies in Patients With Parkinson Disease
- PMID: 37914414
- PMCID: PMC10663029
- DOI: 10.1212/WNL.0000000000207858
Nationwide Retrospective Analysis of Combinations of Advanced Therapies in Patients With Parkinson Disease
Abstract
Background and objectives: Advanced therapies (ATs; deep brain stimulation [DBS] or pump therapies: continuous subcutaneous apomorphine infusion [CSAI], levodopa/carbidopa intestinal gel [LCIG]) are used in later stages of Parkinson disease (PD). However, decreasing efficacy over time and/or side effects may require an AT change or combination in individual patients. Current knowledge about changing or combining ATs is limited to mostly retrospective and small-scale studies. The nationwide case collection Combinations of Advanced Therapies in PD assessed simultaneous or sequential AT combinations in Germany since 2005 to analyze their clinical outcome, their side effects, and the reasons for AT modifications.
Methods: Data were acquired retrospectively by modular questionnaires in 22 PD centers throughout Germany based on clinical records and comprised general information about the centers/patients, clinical (Mini-Mental Status Test/Montréal Cognitive Assessment, Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale [MDS-UPDRS], side effects, reasons for AT modification), and therapeutical (ATs with specifications, oral medication) data. Data assessment started with initiation of the second AT.
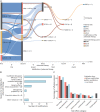
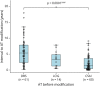
Results: A total of 148 AT modifications in 116 patients were associated with significantly improved objective (median decrease of MDS-UPDRS Part III 4.0 points [p < 0.001], of MDS-UPDRS Part IV 6.0 points [p < 0.001], of MDS-UPDRS Part IV-off-time item 1.0 points [p < 0.001]) and subjective clinical outcome and decreasing side effect rates. Main reasons for an AT modification were insufficient symptom control and side effects of the previous therapy. Subgroup analyses suggest addition of DBS in AT patients with leading dyskinesia, addition of LCIG for leading other cardinal motor symptoms, and addition of LCIG or CSAI for dominant off-time. The most long-lasting therapy-until requiring a modification-was DBS.
Discussion: Changing or combining ATs may be beneficial when 1 AT is insufficient in efficacy or side effects. The outcome of an AT combination is comparable with the clinical benefit by introducing the first AT. The added AT should be chosen dependent on dominant clinical symptoms and adverse effects. Furthermore, prospective trials are needed to confirm the results of this exploratory case collection.
Classification of evidence: This study provides Class IV evidence that, in patients with PD, changing or combining ATs is associated with an improvement in the MDS-UPDRS or subjective symptom reporting.
© 2023 American Academy of Neurology.
Conflict of interest statement
D. Pürner: congress fee from CSL Behring. M.T. Barbe: speaker honoraria from Medtronic, Boston Scientific, Abbot, GE Medical, UCB, Apotherkerverband Köln e.V., Bial; research funding from the Felgenhauer-Stiftung, Foschungspool Klinische Studien (University of Cologne), Horizon 2020 (Gondola), Medtronic (ODIS), Boston Scientific; advisory honoraria for the IQWIG. H. Jergas: personal fees from Boston Scientific. T. Prell: grant from Bundesministerium für Bildung und Forschung (BMBF); honoraria from Abbvie. Licher. E. Gülke: travel grants and honoraria for lectures from Abbvie, Abbott, Medtronic, Bial, Grifols, Kreiskrankenhaus Gummersbach, Zambon/Weser GmBH; co-editor of Thieme journal “Neurologieup2date.” M. Pötter-Nerger: speaker honoraria and study reimbursement from Abbvie, Abbott, Medtronic, Boston Scientific, Licher, Zambon. B. Falkenburger: grants from German Research Foundation (DFG), the German Society of Parkinson's disease (dpv); speaker honoraria from Bial, Stadapharm, PD Neurotechnology, UCB, Zambon. A.M. Jochim: speaker honoraria and online congress fee from Abbvie. M. Rijntjes: advisory boards of Stadapharm, Abbvie, Zambon. C. van Riesen: advisory board and consultancies for Abbvie, Zambon. M. Wolz: honoraria for presentations/advisory boards/consultations from Abbvie, Stadapharm, Zambon, Bial, Teva, Desitin, UCG. G. Ebersbach: honoraria for advisory boards and consultancy from Abbvie, Bial, Desitin, Stada, Esteve Pharma; speaker honoraria from Abbvie, Bial, Britannia Pharma, Desitin, Esteve Pharma, Licher, Stadapharm, Zambon; royalties from Kohlhammer Verlag and Thieme Verlag. S. Paschen: lecture fees from Medtronic; lecture fees and educational grants from Boston Scientific; lecture fees from Insightec. V. Rozanski: travel grants from Teva, Novartis. S. Bornmann: advisory boards of Zambon, Bial. A. Flöel: honoraria/expenses from Roche, Novartis, Biogen, Boehringer-Ingelheim; advisory boards/consulting for Roche, Novartis, Biogen, Bayer. P. Krause: advisory board Medtronic; speaker honoraria Stadapharm, Abbvie. A.A. Kühn: advisory board/honoraria from Boston Scientific, Medtronic. I. Csoti: speaker and advisory board honoraria from Bial, Roche, UCB, Kyowa Kirin. W.H. Jost: speaker and advisory board honoraria from Abbvie, Desitin, Merz, Stadapharm, UCB, Zambon. J. Koschel: travel grant from Desitin; advisory board of Abbvie. P. Lingor: honoraria for advisory boards and consultancies from Stada, Abbvie, Alexion, Bial, ITF Pharma, Desitin, Woolsey Pharma. The remaining authors report no disclosures. Go to
Figures



References
-
- Antonini A, Stoessl AJ, Kleinman LS, et al. . Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson's disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063-2073. doi:10.1080/03007995.2018.1502165 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
