Regorafenib in patients with advanced Ewing sarcoma: results of a non-comparative, randomised, double-blind, placebo-controlled, multicentre Phase II study
- PMID: 37914801
- PMCID: PMC10703915
- DOI: 10.1038/s41416-023-02413-9
Regorafenib in patients with advanced Ewing sarcoma: results of a non-comparative, randomised, double-blind, placebo-controlled, multicentre Phase II study
Abstract
Background: The REGOBONE multi-cohort study explored the efficacy and safety of regorafenib for patients with advanced bone sarcomas; this report details the Ewing sarcoma (ES) cohort.
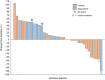
Methods: Patients with relapsed ES progressing despite prior standard therapy, were randomised (2:1) to receive regorafenib or placebo. Patients on placebo could crossover to receive regorafenib after centrally confirmed progression. The primary endpoint was the progression-free rate at 8 weeks. With one-sided α of 0.05, and 80% power, at least 14/24 progression-free patients at 8 weeks were needed for success.
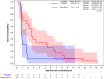
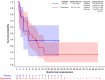
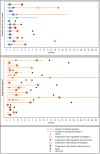
Results: From September 2014 to November 2019, 41 patients were accrued. 36 patients were evaluable for efficacy: 23 on regorafenib and 13 on placebo. Thirteen patients (56%; one-sided 95% CI [37.5%-[)) were progression-free at 8 weeks on regorafenib vs. 1 (7.7%; 95% CI [0.4%-[) on placebo. Median PFS was 11.4 weeks on regorafenib, and 3.9 weeks on placebo. Ten placebo patients crossed over to receive regorafenib after progression. The most common grade ≥3 regorafenib-related adverse events were pain (22%), asthenia (17%), thrombocytopenia (13%) and diarrhoea (13%).
Conclusion: Although the primary endpoint was not met statistically in this randomised cohort, there is evidence to suggest that regorafenib might modestly delay tumour progression in relapsed ES after failure of prior chemotherapy.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
FD received travel grants from Pharmamar, and Leo Pharma, attended advisory boards for Bayer, Lilly. CC attended advisory boards for Leo Pharma, JYB receives research support and honoraria from Eisei, Eli Lilly, MSD, BMS, GSK, Ignyta, Novartis, Pharmamar and Roche unrelated to this work. AL, AI, PB, EK, NP, CP, VL, EB, ESBB, FB, CS, VL, MC, CS,LM, VV, NG and SC declare no competing interests.
Figures
References
-
- Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, et al. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of Sarcoma Alliance for Research Through Collaboration study 003. Oncologist. 2012;17:321. doi: 10.1634/theoncologist.2010-0265. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

