A Retrospective Analysis of Long-Term Prophylaxis with Berotralstat in Patients with Hereditary Angioedema and Acquired C1-Inhibitor Deficiency-Real-World Data
- PMID: 37914894
- PMCID: PMC10847220
- DOI: 10.1007/s12016-023-08972-2
A Retrospective Analysis of Long-Term Prophylaxis with Berotralstat in Patients with Hereditary Angioedema and Acquired C1-Inhibitor Deficiency-Real-World Data
Abstract
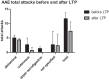
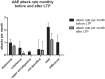
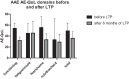
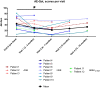
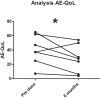
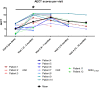
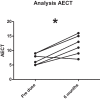
Hereditary angioedema (HAE) and acquired C1-inhibitor deficiency (AAE-C1-INH) are orphan diseases. Berotralstat is a recently licensed long-term prophylaxis (LTP) and the first oral therapy for HAE patients. No approved therapies exist for AAE-C1-INH patients. This study is the first to report real-world clinical data of patients with AAE-C1-INH and HAE who received Berotralstat. All patients treated with Berotralstat were included in this retrospective, bi-centric study. Data was collected from patients' attack calendars and the angioedema quality of life (AE-QoL) and angioedema control test (AECT) questionnaires before treatment, and at 3, 6, and 12 months after treatment and was then analyzed. Twelve patients were included, 3 patients with AAE-C1-INH, 7 patients with HAE type I, and 2 patients with HAE-nC1-INH. One patient (HAE I) quit treatment. Berotralstat was associated with fewer attacks in all groups. After 6 months of treatment, a median decrease of attacks per month was noted for HAE type I patients (3.3 to 1.5) and AAE-C1-INH patients (2.3 to 1.0). No aerodigestive attacks were noted for AAE-C1-INH patients. For HAE-nC1-INH patients, a mean decrease from 3.8 to 1.0 was noted (3 months). For HAE I patients, the total AE-QoL lowered a mean of 24.1 points after 6 months, for HAE-nC1-HAE patients 8.0 points, and for AAE-C1-INH patients 13.7 points. AECT scores increased for HAE I patients (mean: 7.1), HAE-nC1-INH patients (9.0), and AAE-C1-INH patients (4.2) after 6 months. Patients with HAE, HAE-nC1-INH, and AAE-C1-INH treated with Berotralstat showed reduced angioedema attacks and improved AE-QoL and AECT scores.
Keywords: AAE-C1-INH; Acquired angioedema; Berotralstat; Bradykinin; C1-INH-deficiency; HAE; Hereditary angioedema; LTP; Long-term prophylaxis; Prophylaxis.
© 2023. The Author(s).
Conflict of interest statement
No financial assistance from any pharmaceutical company was obtained for this study. The authors of this study declare the following conflict of interests: F. Johnson: has received grant research support and/or speaker/consultancy fees from CSL Behring, Takeda and Pharming. He has also received funding to attend conferences/educational events from CSL Behring, Takeda and Pharming. He has participated as an investigator in a clinical trial/registry for CSL Behring, BioCryst, IONIS Pharmaceuticals and Takeda. A. Stenzl: has no conflict of interest to declare regarding this publication B. Hofauer: has participated as an investigator in clinical trials for CSL Behring and Takeda. H. Heppt: has no conflict of interest to declare regarding this publication E. Ebert: has received grant research support and/or speaker/consultancy fees from CSL Behring and Takeda. She has also received funding to attend conferences/educational events from CSL Behring, Takeda and Pharming. She has participated as an investigator in a clinical trial/registry for CSL Behring, BioCryst, IONIS Pharmaceuticals and Takeda. J. Hahn: has received speaker/consultancy fees from CSL Behring and Takeda. She has also received funding to attend conferences/educational events from CSL Behring and Takeda. She has participated as an investigator in a clinical trial/registry for CSL Behring, BioCryst, Pharvaris and Takeda. J. Greve: has received speaker/consultancy fees from CSL Behring, Takeda, Kalvista and BioCryst. He has also received funding to attend conferences/educational events from CSL Behring and Takeda. He has participated as an investigator in a clinical trial/registry for CSL Behring, BioCryst and Takeda. B. Wollenberg: has no conflict of interest to declare regarding this publication R. Lochbaum: Robin Lochbaum has received consultancy fees from BioCryst and Takeda. He has also received funding to attend conferences/educational events from CSL Behring, BioCryst and Takeda. He has participated as an investigator in a clinical trial/registry for Takeda and Pharvaris. S. Trainotti: has received grant research support and/or speaker/consultancy fees from CSL Behring and Takeda. She has also received funding to attend conferences/educational events from CSL Behring and Takeda. She has participated as an investigator in a clinical trial/registry for CSL Behring, BioCryst, IONIS Pharmaceuticals and Takeda.
Figures
References
-
- Kivity S. New perspectives in acquired angioedema. Isr Med Assoc J IMAJ. 2014;16:313–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

