Vagal sensory neurons mediate the Bezold-Jarisch reflex and induce syncope
- PMID: 37914931
- PMCID: PMC10632149
- DOI: 10.1038/s41586-023-06680-7
Vagal sensory neurons mediate the Bezold-Jarisch reflex and induce syncope
Abstract
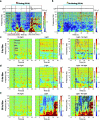
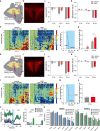
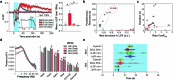
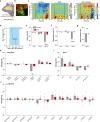
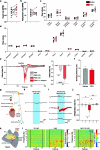
Visceral sensory pathways mediate homeostatic reflexes, the dysfunction of which leads to many neurological disorders1. The Bezold-Jarisch reflex (BJR), first described2,3 in 1867, is a cardioinhibitory reflex that is speculated to be mediated by vagal sensory neurons (VSNs) that also triggers syncope. However, the molecular identity, anatomical organization, physiological characteristics and behavioural influence of cardiac VSNs remain mostly unknown. Here we leveraged single-cell RNA-sequencing data and HYBRiD tissue clearing4 to show that VSNs that express neuropeptide Y receptor Y2 (NPY2R) predominately connect the heart ventricular wall to the area postrema. Optogenetic activation of NPY2R VSNs elicits the classic triad of BJR responses-hypotension, bradycardia and suppressed respiration-and causes an animal to faint. Photostimulation during high-resolution echocardiography and laser Doppler flowmetry with behavioural observation revealed a range of phenotypes reflected in clinical syncope, including reduced cardiac output, cerebral hypoperfusion, pupil dilation and eye-roll. Large-scale Neuropixels brain recordings and machine-learning-based modelling showed that this manipulation causes the suppression of activity across a large distributed neuronal population that is not explained by changes in spontaneous behavioural movements. Additionally, bidirectional manipulation of the periventricular zone had a push-pull effect, with inhibition leading to longer syncope periods and activation inducing arousal. Finally, ablating NPY2R VSNs specifically abolished the BJR. Combined, these results demonstrate a genetically defined cardiac reflex that recapitulates characteristics of human syncope at physiological, behavioural and neural network levels.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





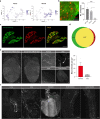
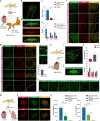

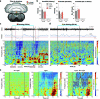
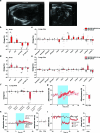





Comment in
-
Explaining how a cardiac reflex causes syncope.Nat Rev Cardiol. 2024 Jan;21(1):9. doi: 10.1038/s41569-023-00961-4. Nat Rev Cardiol. 2024. PMID: 37964079 No abstract available.
References
-
- Von Bezold A. Uber die physiologischen Wirkungen des essigsauren Veratrines. Untersch. Physiolog. Lab. Wurzburg. 1867;1:75–156.
-
- Silvani A, Calandra-Buonaura G, Dampney RAL, Cortelli P. Brain–heart interactions: physiology and clinical implications. Philos. Trans. Royal Soc. A Math. Phys. Eng. Sci. 2016;374:20150181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

