The transcriptional regulatory module CsHB5-CsbZIP44 positively regulates abscisic acid-mediated carotenoid biosynthesis in citrus (Citrus spp.)
- PMID: 37915111
- PMCID: PMC10893943
- DOI: 10.1111/pbi.14219
The transcriptional regulatory module CsHB5-CsbZIP44 positively regulates abscisic acid-mediated carotenoid biosynthesis in citrus (Citrus spp.)
Abstract
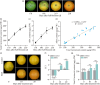
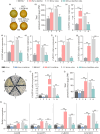
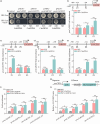
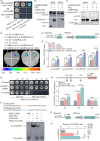
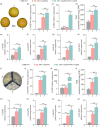
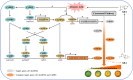
Carotenoids contribute to fruit coloration and are valuable sources of provitamin A in the human diet. Abscisic acid (ABA) plays an essential role in fruit coloration during citrus fruit ripening, but little is known about the underlying mechanisms. Here, we identified a novel bZIP transcription activator called CsbZIP44, which serves as a central regulator of ABA-mediated citrus carotenoid biosynthesis. CsbZIP44 directly binds to the promoters of four carotenoid metabolism-related genes (CsDXR, CsGGPPs, CsBCH1 and CsNCED2) and activates their expression. Furthermore, our research indicates that CsHB5, a positive regulator of ABA and carotenoid-driven processes, activates the expression of CsbZIP44 by binding to its promoter. Additionally, CsHB5 interacts with CsbZIP44 to form a transcriptional regulatory module CsHB5-CsbZIP44, which is responsive to ABA induction and promotes carotenoid accumulation in citrus. Interestingly, we also discover a positive feedback regulation loop between the ABA signal and carotenoid biosynthesis mediated by the CsHB5-CsbZIP44 transcriptional regulatory module. Our findings show that CsHB5-CsbZIP44 precisely modulates ABA signal-mediated carotenoid metabolism, providing an effective strategy for quality improvement of citrus fruit and other crops.
Keywords: CsHB5; CsbZIP44; abscisic acid (ABA); carotenoid; citrus; transcriptional regulatory module.
© 2023 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
All authors have no conflict of interest to declare.
Figures

References
-
- Adams‐Phillips, L. , Barry, C. and Giovannoni, J. (2004) Signal transduction systems regulating fruit ripening. Trends Plant Sci. 9, 331–338. - PubMed
-
- An, J.‐P. , Yao, J.‐F. , Xu, R.‐R. , You, C.‐X. , Wang, X.‐F. and Hao, Y.‐J. (2018) Apple bZIP transcription factor MdbZIP44 regulates abscisic acid‐promoted anthocyanin accumulation. Plant Cell Environ. 41, 2678–2692. - PubMed
-
- An, J.‐P. , Zhang, X.‐W. , Liu, Y.‐J. , Wang, X.‐F. , You, C.‐X. and Hao, Y.‐J. (2021) ABI5 regulates ABA‐induced anthocyanin biosynthesis by modulating the MYB1‐bHLH3 complex in apple. J. Exp. Bot. 72, 1460–1472. - PubMed
-
- Bhagat, P.K. , Verma, D. , Sharma, D. and Sinha, A.K. (2021) HY5 and ABI5 transcription factors physically interact to fine tune light and ABA signaling in Arabidopsis. Plant Mol. Biol. 107, 117–127. - PubMed
-
- Carmona, L. , Zacarías, L. and Rodrigo, M.J. (2012) Stimulation of coloration and carotenoid biosynthesis during postharvest storage of ‘Navelina’ orange fruit at 12°C. Postharvest Biol. Technol. 74, 108–117.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

