Third-generation lentiviral gene therapy rescues function in a mouse model of Usher 1B
- PMID: 37915173
- PMCID: PMC10727968
- DOI: 10.1016/j.ymthe.2023.10.018
Third-generation lentiviral gene therapy rescues function in a mouse model of Usher 1B
Abstract
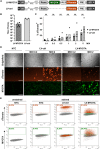
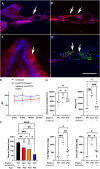
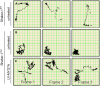
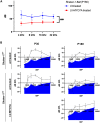
Usher syndrome 1B (USH1B) is a devastating genetic disorder with congenital deafness, loss of balance, and blindness caused by mutations in the myosin-VIIa (MYO7A) gene, for which there is currently no cure. We developed a gene therapy approach addressing the vestibulo-cochlear deficits of USH1B using a third-generation, high-capacity lentiviral vector system capable of delivering the large 6,645-bp MYO7A cDNA. Lentivirally delivered MYO7A and co-encoded dTomato were successfully expressed in the cochlear cell line HEI-OC1. In normal-hearing mice, both cochlea and the vestibular organ were efficiently transduced, and ectopic MYO7A overexpression did not show any adverse effects. In Shaker-1 mice, an USH1B disease model based on Myo7a mutation, cochlear and vestibular hair cells, the main inner ear cell types affected in USH1B, were successfully transduced. In homozygous mutant mice, delivery of MYO7A at postnatal day 16 resulted in a trend for partial recovery of auditory function and in strongly reduced balance deficits. Heterozygous mutant mice were found to develop severe hearing loss at 6 months of age without balance deficits, and lentiviral MYO7A gene therapy completely rescued hearing to wild-type hearing thresholds. In summary, this study demonstrates improved hearing and balance function through lentiviral gene therapy in the inner ear.
Keywords: MYO7A; Usher disease; Usher1B; balance loss; gene therapy; hearing loss; lentiviral vector; unconventional myosin.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.S. is a shareholder in Rescue Hearing Inc. and on the advisory board of MedEl GmbH. J.W.S., M.M., H.B., A.W., A.S., and H.S. have submitted patent applications for lentiviral gene therapy for the inner ear.
Figures





References
-
- Kros C.J., Marcotti W., van Netten S.M., Self T.J., Libby R.T., Brown S.D.M., Richardson G.P., Steel K.P. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat. Neurosci. 2002;5:41–47. - PubMed
-
- Weil D., Küssel P., Blanchard S., Lévy G., Levi-Acobas F., Drira M., Ayadi H., Petit C. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat. Genet. 1997;16:191–193. - PubMed
-
- Géléoc G.G.S., El-Amraoui A. Disease mechanisms and gene therapy for Usher syndrome. Hear. Res. 2020;394 - PubMed
-
- Testa F., Melillo P., Bonnet C., Marcelli V., de Benedictis A., Colucci R., Gallo B., Kurtenbach A., Rossi S., Marciano E., et al. Clinical Presentation and Disease Course of Usher Syndrome Because of Mutations in Myo7a or Ush2a. Retina. 2017;37:1581–1590. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

