Application of the model-informed drug development paradigm to datopotamab deruxtecan dose selection for late-stage development
- PMID: 37915242
- PMCID: PMC10787203
- DOI: 10.1002/psp4.13058
Application of the model-informed drug development paradigm to datopotamab deruxtecan dose selection for late-stage development
Abstract
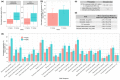
To replace the conventional maximum tolerated dose (MTD) approach, a paradigm for dose optimization and dose selection that relies on model-informed drug development (MIDD) approaches has been proposed in oncology. Here, we report our application of an MIDD approach during phase I to inform dose selection for the late-stage development of datopotamab deruxtecan (Dato-DXd). Dato-DXd is a TROP2-directed antibody-drug conjugate being developed for advanced/metastatic non-small cell lung cancer (NSCLC) and other tumors. Data on pharmacokinetics (PKs), efficacy, and safety in NSCLC were collected in the TROPION-PanTumor01 phase I dose-expansion and -escalation study over a wide dose range of 0.27-10 mg/kg administered every 3 weeks. Population PK and exposure-response analyses were performed iteratively at three data cutoffs to inform dose selection. The 6 mg/kg dose was identified as the optimal dose by the second data cutoff analysis and confirmed by the subsequent third data cutoff analysis. The 6 mg/kg dose was more tolerable (i.e., lower rates of interstitial lung disease, stomatitis, and mucosal inflammation) than the MTD (8 mg/kg) and was more efficacious than 4 mg/kg (simulated mean objective response rate: 23.8% vs. 18.6%; mean hazard ratio of progression-free survival: 0.74) - a candidate dose studied just below 6 mg/kg. Therefore, the 6 mg/kg dose was judged to afford the optimal benefit-risk balance. This case study demonstrated the utility of an MIDD approach for dose optimization and dose selection.
© 2023 Daiichi Sankyo, Inc. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
H.Z.‐G., Y.L., Y.H., A.P., D.S., and N.T. are paid employees of Daiichi Sankyo and own stock in Daiichi Sankyo. J.G., D.S., and P.V. own stock in Daiichi Sankyo. H.L. and D.W. are employees of QuanTx Consulting who provided services to Daiichi Sankyo for this study. S.L. has received support from Daiichi Sankyo and owns stock in Daiichi Sankyo. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Blumenthal G, Jain L, Loeser A, et al. Optimizing dosing in oncology drug development . 2011. https://friendsofcancerresearch.org/wp‐content/uploads/Optimizing_Dosing... Accessed April 28, 2022.
-
- Shah M, Rahman A, Theoret MR, Pazdur R. The drug‐dosing conundrum in oncology – when less is more. N Engl J Med. 2021;385:1445‐1447. - PubMed
-
- Food and Drug Administration . Project Optimus . 2022. https://www.fda.gov/about‐fda/oncology‐center‐excellence/project‐optimus. Accessed September 7, 2022.
-
- Meric‐Bernstam F, Spira AI, Lisber AE, et al. TROPION‐PanTumor01: dose analysis of the TROP2‐directed antibody‐drug conjugate (ADC) datopotamab deruxtecan (Dato‐DXd, DS‐1062) for the treatment (Tx) of advanced or metastatic non‐small cell lung cancer (NSCLC). Presented at American Society of Clinical Oncology annual meeting. June 4–8, 2021. Virtual. J Clin Oncol. 2021;39:9058.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

