The synthetic cannabinoid 5F-MDMB-PICA enhances the metabolic activity and angiogenesis in human brain microvascular endothelial cells by upregulation of VEGF, ANG-1, and ANG-2
- PMID: 37915478
- PMCID: PMC10615825
- DOI: 10.1093/toxres/tfad068
The synthetic cannabinoid 5F-MDMB-PICA enhances the metabolic activity and angiogenesis in human brain microvascular endothelial cells by upregulation of VEGF, ANG-1, and ANG-2
Abstract
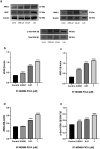
Brain angiogenesis, the formation of new blood vessels from existing brain vasculature, has been previously associated with neural plasticity and addictive behaviors related to substances. Synthetic cannabinoids (SCs) have become increasingly popular due to their ability to mimic the effects of cannabis, offering high potency and easy accessibility. In the current study, we reveal that the SC 5F-MDMB-PICA, the most common SC in the United States in 2019, increases cell metabolic activity and promotes angiogenesis in human brain microvascular endothelial cells (HBMECs). First, we performed an MTT assay to evaluate the effects of 5F-MDMB-PICA treatment at various concentrations (0.0001 μM, 0.001 μM, 0.01 μM, 0.1 μM, and 1 μM) on HBMECs metabolic activity. The results demonstrated higher concentrations of the SC improved cell metabolic activity. Furthermore, 5F-MDMB-PICA treatment enhanced tube formation and migration of HBMECs in a dosage-dependent manner. Additionally, the mRNA, secreted protein, and intracellular protein levels of vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2, which are involved in the regulation of angiogenesis, as well as the protein levels of cannabinoid receptor type-1, were all increased following treatment with 5F-MDMB-PICA. Notably, the phosphorylation levels at Serine 9 residue of glycogen synthase kinase-3β were also increased in the 5F-MDMB-PICA treated HBMECs. Collectively, our findings demonstrate that 5F-MDMB-PICA can enhance angiogenesis in HBMECs, suggesting the significant role of angiogenesis in the response to SCs. Manipulating this interaction may pave the way for innovative treatments targeting SC addiction and angiogenesis-related conditions.
Keywords: 5F-MDMB-PICA; ANG-1 and -2; VEGF; angiogenesis; cannabinoid receptors; synthetic cannabinoids.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







Similar articles
-
Structure-activity relationships for 5F-MDMB-PICA and its 5F-pentylindole analogs to induce cannabinoid-like effects in mice.Neuropsychopharmacology. 2022 Mar;47(4):924-932. doi: 10.1038/s41386-021-01227-8. Epub 2021 Nov 20. Neuropsychopharmacology. 2022. PMID: 34802041 Free PMC article.
-
5F-MDMB-PICA metabolite identification and cannabinoid receptor activity.Drug Test Anal. 2020 Jan;12(1):127-135. doi: 10.1002/dta.2688. Epub 2019 Sep 10. Drug Test Anal. 2020. PMID: 31461219
-
MDMB-FUBINACA Influences Brain Angiogenesis and the Expression of VEGF, ANG-1, and ANG-2.Curr Vasc Pharmacol. 2023;21(5):356-365. doi: 10.2174/1570161121666230913093441. Curr Vasc Pharmacol. 2023. PMID: 37711102
-
Synthesis and in Vitro Cannabinoid Receptor 1 Activity of Recently Detected Synthetic Cannabinoids 4F-MDMB-BICA, 5F-MPP-PICA, MMB-4en-PICA, CUMYL-CBMICA, ADB-BINACA, APP-BINACA, 4F-MDMB-BINACA, MDMB-4en-PINACA, A-CHMINACA, 5F-AB-P7AICA, 5F-MDMB-P7AICA, and 5F-AP7AICA.ACS Chem Neurosci. 2020 Dec 16;11(24):4434-4446. doi: 10.1021/acschemneuro.0c00644. Epub 2020 Nov 30. ACS Chem Neurosci. 2020. PMID: 33253529
-
Detection of the Synthetic Cannabinoids AB-CHMINACA, ADB-CHMINACA, MDMB-CHMICA, and 5F-MDMB-PINACA in Biological Matrices: A Systematic Review.Biology (Basel). 2022 May 23;11(5):796. doi: 10.3390/biology11050796. Biology (Basel). 2022. PMID: 35625524 Free PMC article. Review.
Cited by
-
Cytogenotoxicity and inflammatory response in liver of rats exposed to different doses of cannabis nano emulsions.Arch Toxicol. 2024 Jun;98(6):1877-1890. doi: 10.1007/s00204-024-03712-7. Epub 2024 Mar 17. Arch Toxicol. 2024. PMID: 38494580
-
The Impact of Potent Addictive Substances on Angiogenic Behavior: A Comprehensive Review.Curr Neuropharmacol. 2025;23(5):511-523. doi: 10.2174/1570159X23666240905125037. Curr Neuropharmacol. 2025. PMID: 39248059 Free PMC article. Review.
-
The synthetic cannabinoid 5-fluoro ABICA upregulates angiogenic markers and stimulates tube formation in human brain microvascular endothelial cells.J Taibah Univ Med Sci. 2024 Feb 1;19(2):359-371. doi: 10.1016/j.jtumed.2024.01.002. eCollection 2024 Apr. J Taibah Univ Med Sci. 2024. PMID: 38357583 Free PMC article.
-
Evaluation of the metabolic activity, angiogenic impacts, and GSK-3β signaling of the synthetic cannabinoid MMB-2201 on human cerebral microvascular endothelial cells.J Cannabis Res. 2024 Dec 20;6(1):43. doi: 10.1186/s42238-024-00255-7. J Cannabis Res. 2024. PMID: 39707578 Free PMC article.
-
Effect of EMB-FUBINACA on brain endothelial cell angiogenesis: Expression analysis of angiogenic markers.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):1613-1624. doi: 10.1007/s00210-024-03322-1. Epub 2024 Aug 13. Naunyn Schmiedebergs Arch Pharmacol. 2025. Retraction in: Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):4667. doi: 10.1007/s00210-025-03969-4. PMID: 39136736 Retracted.
References
-
- Lee HS, Han J, Bai HJ, Kim KW. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009:276(17):4622–4635. - PubMed
-
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990:13(5):174–178. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

