Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis
- PMID: 37915501
- PMCID: PMC10617284
- DOI: 10.1177/17562864231207755
Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis
Abstract
Background: Epilepsy is one of the most common chronic brain diseases. Almost one-third of patients have drug-resistant epilepsy (DRE). Cannabidiol is being considered as a potential novel drug for treating DRE.
Objectives: To investigate long-term efficacy and safety of cannabidiol in treatment of DRE and the differences in cannabidiol treatment among patients with different characteristics.
Design: Systematic review and meta-analysis.
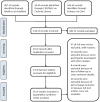
Data sources and methods: Medline, Embase, and CENTRAL were searched for literature. RevMan5.4 was used for meta-analysis. The Intention-to-treat set and the random effect were used as the main analysis. Subgroup analyses were performed according to age, dose, concomitant antiseizure medications (ASMs), epilepsy syndromes, and study designs.
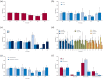
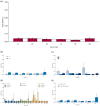
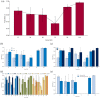
Results: Fifty studies were included in this systematic review. A total of 4791 participants were collected. The responder rates (seizure frequency reduced at least 50%) at 12-, 24-, 48-, 72-, 96-, and 144-week were 0.40 [0.36, 0.45], 0.39 [0.34, 0.44], 0.37 [0.30, 0.44], 0.27 [0.17, 0.37], 0.22 [0.14, 0.30], and 0.38 [0.23, 0.53]. Seizure-free rates were 0.04 [0.03, 0.06], 0.04 [0.03, 0.05], 0.03 [0.02, 0.05], 0.03 [0.02, 0.03], 0.02 [0.01, 0.03], and 0.04 [0.01, 0.06]. Proportion of adverse events were 0.72 [0.61, 0.83], 0.62 [0.42, 0.81], 0.60 [0.41, 0.79], 0.35 [0.14, 0.56], 0.83 [0.75, 0.90], and 0.96 [0.94, 0.99]. The pooled 12-, 24-, 48-, 96-, and 144-week proportion of serious adverse events were 0.15 [0.09, 0.21], 0.23 [0.14, 0.31], 0.10 [0.06, 0.15], 0.31 [0.24, 0.38], and 0.40 [0.35, 0.45]. Subgroup analyses showed that there was no significant difference on efficacy and safety among age subgroups and epilepsy syndromes subgroups. For most periods, there were no significant difference on efficacy among subgroups of dose and concomitant ASMs. However, higher doses and more concomitant ASMs were associated with higher proportion of adverse events.
Conclusion: Cannabidiol treatment of DRE has stable efficacy and fewer adverse events in early period. Long-term use may have decreased efficacy and increased adverse events. Dose escalation may not increase efficacy, but may increase adverse events. Furthermore, cannabidiol use may reduce dosage of other ASMs without reducing efficacy, thereby reducing adverse effects. Cannabidiol may have similar effects in various epilepsy syndromes.
Trial registration: PROSPERO (CRD42022351250).
Keywords: antiseizure medications; drug combination; intractable epilepsy; marijuana.
© The Author(s), 2023.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Clinical efficacy and safety of cannabidiol for pediatric refractory epilepsy indications: A systematic review and meta-analysis.Exp Neurol. 2023 Jan;359:114238. doi: 10.1016/j.expneurol.2022.114238. Epub 2022 Oct 4. Exp Neurol. 2023. PMID: 36206805
-
The efficacy and safety of third-generation antiseizure medications and non-invasive brain stimulation to treat refractory epilepsy: a systematic review and network meta-analysis study.Front Neurol. 2024 Jan 9;14:1307296. doi: 10.3389/fneur.2023.1307296. eCollection 2023. Front Neurol. 2024. PMID: 38264091 Free PMC article.
-
Don't Fear the Reefer-Evidence Mounts for Plant-Based Cannabidiol as Treatment for Epilepsy.Epilepsy Curr. 2019 Mar-Apr;19(2):93-95. doi: 10.1177/1535759719835671. Epilepsy Curr. 2019. PMID: 30955420 Free PMC article.
-
Adjunctive Transdermal Cannabidiol for Adults With Focal Epilepsy: A Randomized Clinical Trial.JAMA Netw Open. 2022 Jul 1;5(7):e2220189. doi: 10.1001/jamanetworkopen.2022.20189. JAMA Netw Open. 2022. PMID: 35802375 Free PMC article. Clinical Trial.
-
The use of cannabidiol as adjunctive therapy in adult patients with drug-resistant epilepsy: a systematic review and meta-analysis.Ther Adv Neurol Disord. 2025 Jan 28;18:17562864251313914. doi: 10.1177/17562864251313914. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 39882324 Free PMC article.
Cited by
-
Use of cannabidiol for off-label treatment of patients with refractory focal, genetic generalised and other epilepsies.Neurol Res Pract. 2025 Jul 22;7(1):49. doi: 10.1186/s42466-025-00408-w. Neurol Res Pract. 2025. PMID: 40696489 Free PMC article.
-
Exploring the efficacy and safety of cannabidiol in individuals with epilepsy: an umbrella review of meta-analyses and systematic reviews.Inflammopharmacology. 2024 Oct;32(5):2987-3005. doi: 10.1007/s10787-024-01523-x. Epub 2024 Aug 21. Inflammopharmacology. 2024. PMID: 39167312
-
The Evolving Role of Cannabidiol-Rich Cannabis in People with Autism Spectrum Disorder: A Systematic Review.Int J Mol Sci. 2024 Nov 20;25(22):12453. doi: 10.3390/ijms252212453. Int J Mol Sci. 2024. PMID: 39596518 Free PMC article.
-
Efficacy and Safety of Cannabis Transdermal Patch for Alleviating Psoriasis Symptoms: Protocol for a Randomized Controlled Trial (CanPatch).Med Cannabis Cannabinoids. 2024 May 30;7(1):99-110. doi: 10.1159/000539492. eCollection 2024 Jan-Dec. Med Cannabis Cannabinoids. 2024. PMID: 39015605 Free PMC article.
-
Research and Clinical Practice Involving the Use of Cannabis Products, with Emphasis on Cannabidiol: A Narrative Review.Pharmaceuticals (Basel). 2024 Dec 6;17(12):1644. doi: 10.3390/ph17121644. Pharmaceuticals (Basel). 2024. PMID: 39770486 Free PMC article. Review.
References
-
- Devinsky O, Vezzani A, O’Brien TJ, et al.. Epilepsy. Nat Rev Dis Primers 2018; 4: 18024–20180503. - PubMed
-
- Chisholm D. and WHO-CHOICE. Cost-effectiveness of first-line antiepileptic drug treatments in the developing world: a population-level analysis. Epilepsia 2005; 46: 751–759. - PubMed
-
- Hwang ST, Stevens SJ, Fu AX, et al.. Intractable generalized epilepsy: therapeutic approaches. Curr Neurol Neurosci Rep 2019; 19: 16–20190226. - PubMed
-
- Lerche H. Drug-resistant epilepsy – time to target mechanisms. Nat Rev Neurol 2020; 16: 595–596. - PubMed
-
- Kwan P, Arzimanoglou A, Berg AT, et al.. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2009; 51: 1069–1077. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

