Tying a knot between crown ethers and porphyrins
- PMID: 37915556
- PMCID: PMC10616700
- DOI: 10.3762/bjoc.19.120
Tying a knot between crown ethers and porphyrins
Abstract
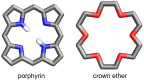
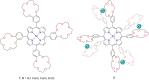
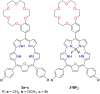
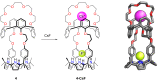
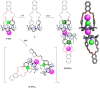
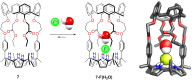
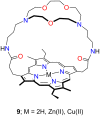
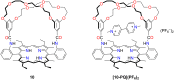
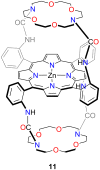
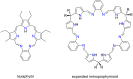
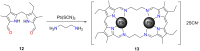
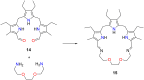
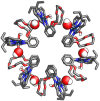
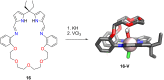
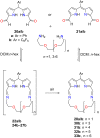
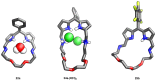
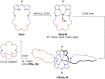
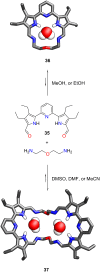
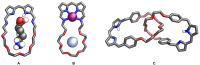
Porphyrins and crown ether hybrids have emerged as a promising class of molecules composed of elements of a tetrapyrrole macrocycle and an oligo(ethylene glycol) segment. These hybrid systems constitute a broad group of compounds, including crowned porphyrins, crownphyrins, and calixpyrrole-crown ether systems forming Pacman complexes with transition metals. Their unique nature accustoms them as excellent ligands and hosts capable of binding guest molecules/ions, but also to undergo unusual transformations, such as metal-induced expansion/contraction. Depending on the design of the particular hybrid, they present unique features involving intriguing redox chemistry, interesting optical properties, and reactivity towards transition metals. In this perspective article, the overview of both the early designs of porphyrin-crown ether hybrids, as well as the most recent advances in the synthesis and characterisation of this remarkable group of macrocyclic systems, are addressed. The discussion covers the strategies employed in synthesising these systems, including cyclisation reactions, self-assembly, and their remarkable reactivity. The potential applications of porphyrin-crown ether hybrids are also highlighted. Moreover, the discussion identifies the challenges associated with synthesising and characterising hybrids, outlining the possible future directions.
Keywords: crown ethers; porphyrinoids; self-assembly; sensors; supramolecular chemistry.
Copyright © 2023, Matviyishyn and Szyszko.
Figures

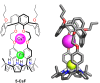

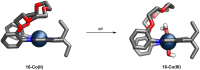
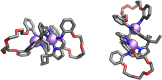
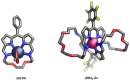

References
LinkOut - more resources
Full Text Sources
