Satralizumab as an add-on treatment in refractory pediatric AQP4-antibody-positive neuromyelitis optica spectrum disorder: a case report
- PMID: 37915570
- PMCID: PMC10616785
- DOI: 10.3389/fimmu.2023.1257955
Satralizumab as an add-on treatment in refractory pediatric AQP4-antibody-positive neuromyelitis optica spectrum disorder: a case report
Abstract
Neuromyelitis optica spectrum disorder (NMOSD) is a rare autoimmune disease of the central nervous system. Relapse and incomplete recovery from relapse are common in NMOSD. Most patients with NMOSD have IgG to aquaporin-4 (AQP4-IgG). New biological agents for AQP4-IgG-seropositive NMOSD, such as satralizumab, have become available for maintenance therapy. Satralizumab is an anti-interleukin-6 receptor monoclonal antibody. To date, few studies have evaluated satralizumab as an add-on treatment in pediatric NMOSD patients. Here, we report an 11-year-old girl with NMOSD who frequently relapsed under long-term treatment, including oral prednisone, rituximab, mycophenolate mofetil (MMF), and maintenance intravenous immunoglobulin treatment even with B-cell depletion. For the poor treatment response and to improve the efficacy of relapse prevention further, the patient received satralizumab treatment as an add-on therapy to MMF plus oral prednisone, with a dose of 120 mg administered subcutaneously at weeks 0, 2, and 4 and every 4 weeks after that. After initiating satralizumab, the patient remained relapse-free for 14 months at the last follow-up. Satralizumab might be effective and safe as an add-on treatment in refractory pediatric AQP4-IgG-seropositive NMOSD under B-cell depletion.
Keywords: AQP4; NMOSD; pediatric; relapse; satralizumab.
Copyright © 2023 Li, Wu, Zeng, Wu, Hou, Zhu, Liao, Tian, Chen, Peng and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
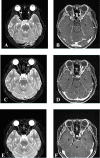
Figures


Similar articles
-
Satralizumab: A Review in Neuromyelitis Optica Spectrum Disorder.CNS Drugs. 2023 Apr;37(4):363-370. doi: 10.1007/s40263-023-00995-9. Epub 2023 Mar 18. CNS Drugs. 2023. PMID: 36933107 Review.
-
Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder.N Engl J Med. 2019 Nov 28;381(22):2114-2124. doi: 10.1056/NEJMoa1901747. N Engl J Med. 2019. PMID: 31774956 Clinical Trial.
-
SAkuraBONSAI: Protocol design of a novel, prospective study to explore clinical, imaging, and biomarker outcomes in patients with AQP4-IgG-seropositive neuromyelitis optica spectrum disorder receiving open-label satralizumab.Front Neurol. 2023 Feb 17;14:1114667. doi: 10.3389/fneur.2023.1114667. eCollection 2023. Front Neurol. 2023. PMID: 36873431 Free PMC article.
-
Satralizumab after inebilizumab treatment in a patient with recurrent neuromyelitis optica spectrum disorder: A case report.Medicine (Baltimore). 2025 Apr 4;104(14):e42067. doi: 10.1097/MD.0000000000042067. Medicine (Baltimore). 2025. PMID: 40193676 Free PMC article.
-
Monoclonal Antibody Therapies Beyond Complement for NMOSD and MOGAD.Neurotherapeutics. 2022 Apr;19(3):808-822. doi: 10.1007/s13311-022-01206-x. Epub 2022 Mar 10. Neurotherapeutics. 2022. PMID: 35267170 Free PMC article. Review.
Cited by
-
Case report: Satralizumab therapy for bilateral refractory optic neuritis following the first dose of bivalent human papilloma virus vaccine.Front Immunol. 2024 Nov 19;15:1499045. doi: 10.3389/fimmu.2024.1499045. eCollection 2024. Front Immunol. 2024. PMID: 39628490 Free PMC article.
-
Research hotspots and emerging topics in neuromyelitis optica spectrum disorder treatment: Insights from a bibliometric analysis.Medicine (Baltimore). 2025 Jun 6;104(23):e42850. doi: 10.1097/MD.0000000000042850. Medicine (Baltimore). 2025. PMID: 40489809 Free PMC article. Review.
-
Visual improvement in a case of neuromyelitis optica spectrum disorder-related optic neuritis after 18 months of treatment with satralizumab: A case report.Heliyon. 2024 Jul 25;10(15):e35142. doi: 10.1016/j.heliyon.2024.e35142. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157378 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

