Novel insight into cGAS-STING pathway in ischemic stroke: from pre- to post-disease
- PMID: 37915571
- PMCID: PMC10616885
- DOI: 10.3389/fimmu.2023.1275408
Novel insight into cGAS-STING pathway in ischemic stroke: from pre- to post-disease
Abstract
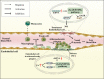
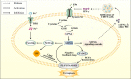
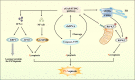
Ischemic stroke, a primary cause of disability and the second leading cause of mortality, has emerged as an urgent public health issue. Growing evidence suggests that the Cyclic GMP-AMP synthase (cGAS)- Stimulator of interferon genes (STING) pathway, a component of innate immunity, is closely associated with microglia activation, neuroinflammation, and regulated cell death in ischemic stroke. However, the mechanisms underlying this pathway remain inadequately understood. This article comprehensively reviews the existing literature on the cGAS-STING pathway and its multifaceted relationship with ischemic stroke. Initially, it examines how various risk factors and pre-disease mechanisms such as metabolic dysfunction and senescence (e.g., hypertension, hyperglycemia, hyperlipidemia) affect the cGAS-STING pathway in relation to ischemic stroke. Subsequently, we explore in depth the potential pathophysiological relationship between this pathway and oxidative stress, endoplasmic reticulum stress, neuroinflammation as well as regulated cell death including ferroptosis and PANoptosis following cerebral ischemia injury. Finally, it suggests that intervention targeting the cGAS-STING pathway may serve as promising therapeutic strategies for addressing neuroinflammation associated with ischemic stroke. Taken together, this review concludes that targeting the microglia cGAS-STING pathway may shed light on the exploration of new therapeutic strategies against ischemic stroke.
Keywords: cGAS-STING pathway; intervention target; ischemic stroke; microglia; neuroinflammation.
Copyright © 2023 Ma, Xin, She, Liu, Ge and Mei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Dawson J, Béjot Y, Christensen LM, De Marchis GM, Dichgans M, Hagberg G, et al. . European Stroke Organisation (ESO) guideline on pharmacological interventions for long-term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur Stroke J (2022) 7:I–II. doi: 10.1177/23969873221100032 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

